Succinyl-CoA
| Names | |
|---|---|
| IUPAC name 4-[(2-{3-[(2R)-4-{[1,3-Dihydroxy-1,3-dioxo-3-(3′-O-phosphonoadenosin-5′-O-yl)-1λ5,3λ5-diphosphoxan-1-yl]oxy}-3,3-dimethylbutanamido]propanamido}ethyl)sulfanyl]-4-oxobutanoic acid | |
| Systematic IUPAC name (9R)-1-[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5,10,14,19-pentaoxo-2,4,6-trioxa-18-thia-11,15-diaza-3λ5,5λ5-diphosphadocosan-22-oic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.009.163 |
| MeSH | succinyl-coenzyme+A |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C25H40N7O19P3S | |
| Molar mass | 867.608 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
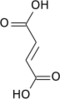
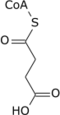
Succinyl-coenzyme A, abbreviated as succinyl-CoA (/ˌsʌksɪnəlˌkoʊˈeɪ/) or SucCoA, is a thioester of succinic acid and coenzyme A.
Sources
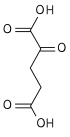
[edit]It is an important intermediate in the citric acid cycle, where it is synthesized from α-ketoglutarate by α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added.
With B12 as an enzymatic cofactor, it is also synthesized from propionyl CoA, the odd-numbered fatty acid, which cannot undergo beta-oxidation.[1] Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12-dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA. Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter the citric acid cycle.
Fate
[edit]It is converted into succinate through the hydrolytic release of coenzyme A by succinyl-CoA synthetase (succinate thiokinase).
Another fate of succinyl-CoA is porphyrin synthesis, where succinyl-CoA and glycine are combined by ALA synthase to form δ-aminolevulinic acid (dALA). This process is the committed step in the biosynthesis of porfobilinogen and thus hemoglobin.
Formation
[edit]Succinyl CoA can be formed from methylmalonyl CoA through the utilization of deoxyadenosyl-B12 (deoxyadenosylcobalamin) by the enzyme methylmalonyl-CoA mutase. This reaction, which requires vitamin B12 as a cofactor, is important in the catabolism of some branched-chain amino acids as well as odd-chain fatty acids.
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
References
[edit]- ^ Halarnkar PP, Blomquist GJ (1989). "Comparative aspects of propionate metabolism". Comp. Biochem. Physiol. B. 92 (2): 227–31. doi:10.1016/0305-0491(89)90270-8. PMID 2647392.


 French
French Deutsch
Deutsch