Vanillate monooxygenase
| vanillate monooxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.14.13.82 | ||||||||
| CAS no. | 39307-11-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
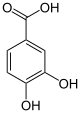
In enzymology, a vanillate monooxygenase (EC 1.14.13.82) is an enzyme that catalyzes the chemical reaction
The 4 substrates of this enzyme are vanillate, O2, NADH, and H+, whereas its 4 products are 3,4-dihydroxybenzoate, NAD+, H2O, and formaldehyde.
This enzyme belongs to the family of oxidoreductases, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with NADH or NADPH as one donor, and incorporation of one atom o oxygen into the other donor. The systematic name of this enzyme class is vanillate:oxygen oxidoreductase (demethylating). Other names in common use include 4-hydroxy-3-methoxybenzoate demethylase, and vanillate demethylase. This enzyme participates in 2,4-dichlorobenzoate degradation.
References
[edit]- Brunel F, Davison J (1988). "Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase". J. Bacteriol. 170 (10): 4924–4930. PMC 211539. PMID 3170489.
- Priefert H, Rabenhorst J, Steinbüchel A (1997). "Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate". J. Bacteriol. 179 (8): 2595–2607. PMC 179009. PMID 9098058.


 French
French Deutsch
Deutsch