Dextrin
 | |
| Identifiers | |
|---|---|
| ChemSpider |
|
| ECHA InfoCard | 100.029.693 |
| E number | E1400 (additional chemicals) |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| Properties | |
| (C6H10O5)n | |
| Molar mass | variable |
| Appearance | white or yellow powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
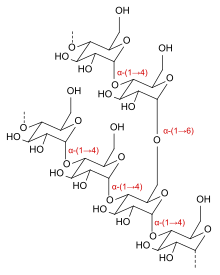
Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch[1] and glycogen.[2] Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds.
Dextrins can be produced from starch using enzymes like amylases, as during digestion in the human body and during malting and mashing in beer brewing[3] or by applying dry heat under acidic conditions (pyrolysis or roasting). This procedure was first discovered in 1811 by Edme-Jean Baptiste Bouillon-Lagrange.[4] The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavor, color and crispness. Dextrins produced by heat are also known as pyrodextrins. Starch hydrolyses during roasting under acidic conditions, and short-chained starch parts partially rebranch with α-(1,6) bonds to the degraded starch molecule.[5] See also Maillard reaction.
Dextrins are white, yellow, or brown powders that are partially or fully water-soluble, yielding optically active solutions of low viscosity. Most of them can be detected with iodine solution, giving a red coloration; one distinguishes erythrodextrin (dextrin that colours red) and achrodextrin (giving no colour).
White and yellow dextrins from starch roasted with little or no acid are called British gum.
Uses
[edit]Yellow dextrins are used as water-soluble glues[6] in remoistenable envelope adhesives and paper tubes, in the mining industry as additives in froth flotation, in the foundry industry as green strength additives in sand casting, as printing thickener for batik resist dyeing, and as binders in gouache paint and also in the leather industry.
White dextrins are used as:
- A crispness enhancer for food processing, in food batters, coatings, and glazes, (INS number 1400)
- a textile finishing and coating agent to increase weight and stiffness of textile fabrics[citation needed]
- a thickening and binding agent in pharmaceuticals and paper coatings[citation needed]
- a pyrotechnic binder and fuel; this is added to firework effect or color compositions; allowing them to solidify as pellets (stars or comets); and to sparkler compositions which the handle is dipped in[citation needed]
- a stabilizing agent for certain explosive metal azides, particularly Lead(II) azide[citation needed]
Owing to their rebranching, dextrins are less digestible than other carbohydrates. Indigestible dextrins have been developed as soluble stand-alone fiber supplements and for adding to processed food products.[7]
Other types
[edit]- Maltodextrin
Maltodextrin is a short-chain starch sugar used as a food additive. It is also produced by enzymatic hydrolysis from gelled starch, and is usually found as a creamy-white hygroscopic spray-dried powder. Maltodextrin is easily digestible, being absorbed as rapidly as glucose, and might either be moderately sweet or have hardly any flavor at all.
- Cyclodextrin
The cyclical dextrins are known as cyclodextrins. They are formed by enzymatic degradation of starch by certain bacteria,[8] for example, Paenibacillus macerans (Bacillus macerans). Cyclodextrins have toroidal structures formed by 6–8 glucose residues.
- Amylodextrin is a linear dextrin or short chained amylose (DP 20-30) that can be produced by enzymatic hydrolysis of the alpha-1,6 glycosidic bonds or debranching amylopectin. Amylodextrin colors blue with iodine.
- (Beta) Limit dextrin is the remaining polymer produced by enzymatic hydrolysis of amylopectin with beta amylase, which cannot hydrolyse the alpha-1,6 bonds at branch points.
- (Alpha) Limit dextrin is a short chained branched amylopectin remnant, produced by hydrolysis of amylopectin with alpha amylase.
- Highly branched cyclic dextrin is a dextrin produced from enzymatic breaking of the amylopectin in clusters and using branching enzyme to form large cyclic chains.[9]
See also
[edit]- Brewing – Process in beer production
- Cellodextrin – Glucose polymers
- Dextrose equivalent – relative sweetness of sugars
- Icodextrin – Pharmaceutical drug
- Modified starch – Thickening agent
- Starch gelatinization – Process of breaking down the intermolecular bonds of starch by water
References
[edit]- ^ An Introduction to the chemistry of plants - Vol II: Metabolic processes, P. Haas and T. G. Hill, London (Longmans, Green & Co.), 1913; pages 123-127
- ^ Salway, JG. Medical Biochemistry at a Glance. Second Edition. Malden, MA (Blackwell Publishing), 2006; page 66
- ^ Michael Lewis, Tom W. Young (2002), "Brewing", Kluwer Academic, ISBN 0-306-47274-0.
- ^ Edme-Jean-Baptiste Bouillon-Lagrange, Revista CENIC Ciencias Biológicas, Vol. 44, No. 1, mayo-agosto, 2013
- ^ Alistair M. Stephen, Glyn O. Phillips, Peter A. Williams (2006), "Food polysaccharides and their applications 2nd edition", p 92-99, CRC Press, Taylor & Francis Group, ISBN 0-8247-5922-2
- ^ Industrial uses of starch and its derivatives. London: Applied Science Publ. 1976. ISBN 0-85334-691-7.
- ^ "Types of Fiber and Their Health Benefits (on WebMD)".
- ^ Wüpper, Svenja; Lüersen, Kai; Rimbach, Gerald (2021-03-09). "Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective". Biomolecules. 11 (3): 401. doi:10.3390/biom11030401. ISSN 2218-273X. PMC 7998733. PMID 33803150.
- ^ T. Hiroki, K. Iwao, T. Noboru, S. Yuji, Y. Mikio, Journal: Seibutsu Kogakkaishi, Vol:84; No:2; Page: 61-66 (2006), Industrial Production of Branching Enzyme and Its Application to Production of Highly Branched Cyclic Dextrin (Cluster Dextrin)[1] Archived 2012-02-29 at the Wayback Machine
External links
[edit]- . Encyclopædia Britannica. Vol. VII (9th ed.). 1878. p. 146.
- Dextrins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- EAFUS


 French
French Deutsch
Deutsch