Zofenoprilat
 | |
| Names | |
|---|---|
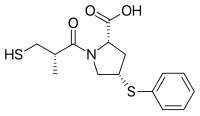
| IUPAC name (4S)-N-[(2S)-2-Methyl-3-sulfanylpropanoyl]-4-(phenylsulfanyl)-L-proline | |
| Systematic IUPAC name (2S,4S)-1-[(2S)-2-Methyl-3-sulfanylpropanoyl]-4-(phenylsulfanyl)pyrrolidine-2-carboxylic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H19NO3S2 | |
| Molar mass | 325.44 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
This article relies largely or entirely on a single source. (October 2022) |
Zofenoprilat is an angiotensin-converting enzyme inhibitor, and is the free sulfhydryl active metabolite of zofenopril.[1]
References
[edit]- ^ Subissi, A; Evangelista, S; Giachetti, A (1999). "Preclinical Profile of Zofenopril: An Angiotensin Converting Enzyme Inhibitor with Peculiar Cardioprotective Properties". Cardiovascular Drug Reviews. 17 (2): 115. doi:10.1111/j.1527-3466.1999.tb00008.x.


 French
French Deutsch
Deutsch