1,1-Difluoroethylene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,1-Difluoroethene | |||
| Other names Difluoro-1,1-ethylene; R-1132a; Halocarbon 1132 A; Freon 1132A; Vinylidene difluoride; Vinylidene fluoride[1] | |||
| Identifiers | |||
3D model (JSmol) | |||
| Abbreviations | VDF | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.789 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1959 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C2H2F2 | |||
| Molar mass | 64.035 g·mol−1 | ||
| Appearance | Colorless gas[2] | ||
| Odor | Slightly ethereal[1] | ||
| Density | 2.89 kg/m3 (vapor, 0 °C)[2] 1.122 g/mL (liquid, -84 °C)[2] | ||
| Melting point | −144 °C (−227 °F; 129 K)[2] | ||
| Boiling point | −84 °C (−119 °F; 189 K)[2] | ||
| 0.254 g/L[3] | |||
| Vapor pressure | 35.2 atm (20°C)[4] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Flammable[4] | ||
| GHS labelling: | |||
 | |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| 380 °C (716 °F; 653 K)[1] | |||
| Explosive limits | 5.5%-21.3%[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | none[4] | ||
REL (Recommended) | TWA 1 ppm C 5 ppm[4] | ||
IDLH (Immediate danger) | N.D.[4] | ||
| Related compounds | |||
Related compounds | 1,2-Difluoroethylene; Fluoroethylene; Trifluoroethylene; 2-Chloro-1,1-difluoroethylene; | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
1,1-Difluoroethylene, also known as vinylidene fluoride, is a hydrofluoroolefin. This colorless, flammable gas is a difluorinated derivative of ethylene. Global production in 1999 was approximately 33,000 metric tons.[3] It is primarily used in the production of fluoropolymers such as polyvinylidene fluoride and FKM.
Preparation
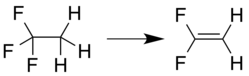
[edit]1,1-Difluoroethylene can be prepared by elimination reaction from a 1,1,1-trihaloethane compound, for example, loss of hydrogen chloride from 1-chloro-1,1-difluoroethane:[5]
or loss of hydrogen fluoride from 1,1,1-trifluoroethane:[6]
See also
[edit]References
[edit]- ^ a b c "Difluoro-1,1-ethylene". Gas Encyclopaedia. Air Liquide. Archived from the original on 2016-03-03. Retrieved 2012-04-23.
- ^ a b c d e Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b "1,1'-Difluoroethylene (VDF,VF2)" (PDF). International Programme on Chemical Safety. Archived from the original (PDF) on 2012-01-04. Retrieved 2012-04-23.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0662". National Institute for Occupational Safety and Health (NIOSH).
- ^ Kirk-Othmer Encyclopedia of Chemical Technology (4 ed.). John Wiley and Sons. 1994. pp. V11. Retrieved 8 July 2017.
- ^ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2000). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_349. ISBN 978-3-527-30385-4.


 French
French Deutsch
Deutsch