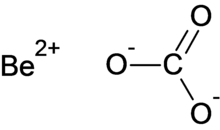
Beryllium carbonate
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.032.740 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UN number | 1566 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| BeCO3 | |
| Molar mass | 69.020 g·mol−1 |
| Melting point | 54 °C (129 °F; 327 K) |
| Boiling point | 100 °C (212 °F; 373 K) decomposes |
| 0.36 g/100 mL | |
| Thermochemistry | |
Heat capacity (C) | 65 J/mol·K[1] |
Std molar entropy (S⦵298) | 52 J/mol·K[1] |
Std enthalpy of formation (ΔfH⦵298) | -1025 kJ/mol[1] |
Gibbs free energy (ΔfG⦵) | -948 kJ/mol[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Toxic (T) Irritant (Xi) |
| GHS labelling:[3] | |
   | |
| Danger | |
| H301, H315, H317, H319, H330, H335, H350i, H372, H411 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 150 mg/kg (guinea pig) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
REL (Recommended) | Ca C 0.0005 mg/m3 (as Be)[2] |
IDLH (Immediate danger) | Ca [4 mg/m3 (as Be)][2] |
| Related compounds | |
Other cations | Magnesium carbonate Calcium carbonate Strontium carbonate Barium carbonate Radium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Beryllium carbonate is a chemical compound with the chemical formula BeCO3.
Structures
[edit]There are three forms reported, anhydrous, tetrahydrate and basic beryllium carbonate. The anhydrous form is reported to be unstable, decomposing to BeO and carbon dioxide, and requiring storage under CO2.[4] The tetrahydrate is said to be formed when CO2 is bubbled through a solution of Be(OH)2 and is also reported to be similarly unstable.[5]
Preparation
[edit]Basic beryllium carbonate is a mixed salt, which can be prepared by the reaction of beryllium sulfate and ammonium carbonate, and contains both carbonate and hydroxide ions, with formula Be2CO3(OH)2.[6] It is believed that in the older literature this is probably what was referred to as beryllium carbonate.[6]
Safety
[edit]It may cause irritation. Toxic. It should be handled carefully since several related beryllium compounds are known carcinogens.
Natural occurrence
[edit]No formations of purely beryllium carbonate are known to occur naturally. The only Be-rich carbonate mineral currently known is niveolanite.[7]
References
[edit]- ^ a b c d "Beryllium carbonate".
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- ^ GHS: GESTIS 082790
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ David Anthony Everest, 1964, The Chemistry of Beryllium, Elsevier Pub. Co.
- ^ a b J.E. Macintyre, Dictionary of Inorganic Compounds 1992 CRC Press ISBN 0-412-30120-2
- ^ "Niveolanite".


 French
French Deutsch
Deutsch