Neodymium(III) carbonate
 | |
| Names | |
|---|---|
| IUPAC name neodymium(3+);tricarbonate | |
| Other names neodymium(III) carbonate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.025.072 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
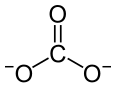
| Nd2(CO3)3 | |
| Molar mass | 468.53 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P321, P362+P364, P403+P233, P405, P501 | |
| Related compounds | |
Other anions | neodymium(III) oxide, neodymium(III) hydroxide |
Other cations | praseodymium(III) carbonate samarium(III) carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion has charge -2.[1] It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red,[2] while the octahydrate is a pink solid.[3] Both of these salts are insoluble in water.[4]
Preparation
[edit]Neodymium(III) carbonate can be created by the reaction between neodymium(III) hydroxide and carbon dioxide:
- 2Nd(OH)3 + 3CO2 → Nd2(CO3)3 + 3H2O
Neodymium(III) carbonate can also be created by passing carbon dioxide under pressure through a solution of neodymium(III) chloride containing aniline:
- 2NdCl3 + 3CO2 + 6C6H2NH2 + H2O → Nd2(CO3)3 + 6C_6H_5NH_2·HCl
It can also be obtained from the hydrolysis of neodymium(III) chloroacetate:[4]
- 2Nd(C2Cl3O2)3 + 3H2O → Nd2(CO3)3 + 6CHCl3 + 3CO2
Another way to obtain neodymium(III) carbonate is by reacting neodymium(III) chloride with ammonium bicarbonate in water.[5]
Properties
[edit]Chemical properties
[edit]Neodymium(III) carbonate dissolves in acids and releases carbon dioxide:
- Nd2(CO3)3 + 6H+ → 2Nd3+ + 3H2O + 3CO2↑
Neodymium(III) carbonate can react with an acid to produce many neodymium salts:
- H+ + Nd2(CO3)3 → Nd + H2O + CO2
For example, to create neodymium acetate with neodymium(III) carbonate:
- 6CH3COOH + 2Nd2(CO3)3 → 2Nd(CH3COO)3 + 3H2O + 3CO2
Neodymium(III) carbonate can form complexes with ammonium carbonate, sodium carbonate and potassium carbonate and many other salts, which explains their greater solubility in aqueous solutions than in distilled water. It can easily be converted into other neodymium compounds, such as neodymium(III) oxide when heated.[6] It can also form compounds with hydrazine, such as Nd2(CO3)3·12N2H4·4H2O which is a transparaent crystal that is slightly soluble in water but insoluble in benzene, d20°C = 1.96 g/cm3.[7]
Physical properties
[edit]Neodymium(III) carbonate forms crystals and has a crystalline hydrate composition of Nd2(CO3)3·n H2O, where n = 2.5 and 8. It doesn't dissolve in water.[3]
Applications
[edit]Neodymium carbonate can be used for lasers, glass coloring and tinting, and dielectrics.[6]
See also
[edit]References
[edit]- ^ See https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium_III_-carbonate-hydrate
- ^ Rare earth elements: Main volume, Phần 3 (Leopold Gmelin; Verlag Chemie, 1994), page 22; 68. Retrieved 4 February 2021.
- ^ a b Handbook… (Pierre Villars, Karin Cenzual, Roman Gladyshevskii; Walter de Gruyter GmbH & Co KG, 24 thg 7, 2017 - 1970 pages), page 999. Retrieved 4 February 2021.
- ^ a b 《无机化学丛书》. 第七卷 钪 稀土元素. 易宪武 黄春晖 等编.科学出版社. tr. 174, 碳酸盐.ISBN 978-7-03-030574-9
- ^ 黄婷. 碳酸钇、碳酸钕的结晶及相关技术研究[J]. 《南昌大学》.2005年
- ^ a b "Neodymium Carbonate".
- ^ Uchenye zapiski: Serii︠a︡ khimicheskikh nauk (S.M. Kirov adyna Azărbai̐jan Dȯvlăt Universiteti; 1975). Retrieved 7 February 2021.


 French
French Deutsch
Deutsch