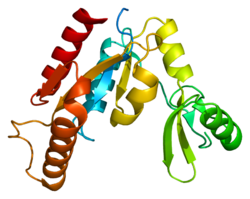
CASK
Peripheral plasma membrane protein CASK is a protein that in humans is encoded by the CASK gene.[5][6] This gene is also known by several other names: CMG 2 (CAMGUK protein 2), calcium/calmodulin-dependent serine protein kinase 3 and membrane-associated guanylate kinase 2. CASK gene mutations are the cause of XL-ID with or without nystagmus and MICPCH, an X-linked neurological disorder.
Gene
[edit]This gene is located on the short arm of the X chromosome (Xp11.4). It is 404,253 bases in length and lies on the Crick (minus) strand. The encoded protein has 926 amino acids with a predicted molecular weight of 105,123 daltons.
Function
[edit]This protein is a multidomain scaffolding protein with a role in synaptic transmembrane protein anchoring and ion channel trafficking. It interacts with the transcription factor TBR1 and binds to several cell-surface proteins including neurexins and syndecans.
Clinical importance
[edit]This gene has been implicated in X-linked mental retardation,[7] including specifically mental retardation and microcephaly with pontine and cerebellar hypoplasia.[8] The role of CASK in disease is primarily associated with a loss of function (under expression) of the CASK gene as a result of a deletion, missense or splice mutation.[9] It appears that mutations in the gene lead to diminished amounts of the protein being coded. As a result, CASK is unable to form complexes with other proteins leading to a cascade of events. Research has shown there is significant down-regulation of the genes involved in pre-synaptic development and of CASK protein interactors.[10]
Males affected by CASK variants tend to have more severe symptoms than females due to the X-linked nature of the disease. These genetic issues are often fatal in the womb for male embryos[11][12] or else lead to infant mortality. Females with CASK mutations have variable phenotypes with moderate to severe intellectual disability. CASK missense mutations and some splice mutations can lead to the milder neurodevelopmental phenotype.[12]
CASK related disorders are mainly found in girls. The prevalence is unknown but generally thought to be below 400 cases worldwide. Patients are often born healthy but within the first few months of life show progressive microcephaly. Although there can be prenatal deceleration of head circumference growth, the majority of cases will not be diagnosed according to current recommendations for fetal CNS routine assessment.[13]
The exact mode of pathology is not clear, but evidence from mice models indicates CASK deficiency in neurones causes the following effects:[14]
- reduced levels of associated proteins such as Mint1[15] and neurexin
- Higher levels of Neuroligin 1
- Increased glutamate release at synapses and reduced GABA release affecting the E/I balance in maturing neural circuits[16]
- Down-regulation of GluN2B resulting in disruption of synaptic E/I balance[17]
Even slight changes in CASK expression in humans leads to dysregulation of the formation of presynapses, especially in inhibitory neurones.[10]
Interactions
[edit]CASK has been shown to interact with:
- KCNJ4[18][19]
- APBA1[20][21]
- ATP2B4[22]
- CINAP and TBR1[23]
- DLG1[19][24][25]
- DLG4[24]
- F11 receptor[26][27]
- ID1[28]
- KCNJ12[18][19]
- LIN7A[19][20]
- Nephrin[29]
- Parkin (ligase)[30]
- RPH3A[31]
- SDC2[31][32]
External links
[edit]- Project CASK - A non-profit based in the United States driving research breakthroughs to treat and cure CASK-related disorders.
- CASK Research Foundation - A non-profit based in the UK for research, information and support into CASK related disorders including MICPCH
- Angelina CASK Neurological Research Foundation - A non-profit based in Australia creating research grants for research into CASK gene related disorders.
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000147044 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031012 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Dimitratos SD, Stathakis DG, Nelson CA, Woods DF, Bryant PJ (July 1998). "The location of human CASK at Xp11.4 identifies this gene as a candidate for X-linked optic atrophy". Genomics. 51 (2): 308–309. doi:10.1006/geno.1998.5404. PMID 9722958.
- ^ "Entrez Gene: CASK Calcium/calmodulin-dependent serine protein kinase (MAGUK family)".
- ^ Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, et al. (May 2009). "A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation". Nature Genetics. 41 (5): 535–543. doi:10.1038/ng.367. PMC 2872007. PMID 19377476.
- ^ Burglen L, Chantot-Bastaraud S, Garel C, Milh M, Touraine R, Zanni G, et al. (March 2012). "Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient". Orphanet Journal of Rare Diseases. 7 (18): 18. doi:10.1186/1750-1172-7-18. PMC 3351739. PMID 22452838.
- ^ Hackett A, Tarpey PS, Licata A, Cox J, Whibley A, Boyle J, et al. (May 2010). "CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes". European Journal of Human Genetics. 18 (5): 544–552. doi:10.1038/ejhg.2009.220. PMC 2987321. PMID 20029458.
- ^ a b Becker M, Mastropasqua F, Reising JP, Maier S, Ho ML, Rabkina I, et al. (September 2020). "Presynaptic dysfunction in CASK-related neurodevelopmental disorders". Translational Psychiatry. 10 (1): 312. doi:10.1038/s41398-020-00994-0. PMC 7490425. PMID 32929080.
- ^ Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, et al. (September 2008). "Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum". Nature Genetics. 40 (9): 1065–1067. doi:10.1038/ng.194. PMID 19165920. S2CID 91094953.
- ^ a b Moog U, Bierhals T, Brand K, Bautsch J, Biskup S, Brune T, et al. (April 2015). "Phenotypic and molecular insights into CASK-related disorders in males". Orphanet Journal of Rare Diseases. 10 (1): 44. doi:10.1186/s13023-015-0256-3. PMC 4449965. PMID 25886057.
- ^ Gafner M, Boltshauser E, D'Abrusco F, Battini R, Romaniello R, D'Arrigo S, et al. (September 2022). "Expanding the natural history of CASK-related disorders to the prenatal period". Developmental Medicine and Child Neurology. 65 (4): 544–550. doi:10.1111/dmcn.15419. hdl:11568/1157845. PMID 36175354. S2CID 252622483.
- ^ Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, et al. (February 2007). "Deletion of CASK in mice is lethal and impairs synaptic function". Proceedings of the National Academy of Sciences of the United States of America. 104 (7): 2525–2530. Bibcode:2007PNAS..104.2525A. doi:10.1073/pnas.0611003104. PMC 1892970. PMID 17287346.
- ^ Butz S, Okamoto M, Südhof TC (September 1998). "A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain". Cell. 94 (6): 773–782. doi:10.1016/S0092-8674(00)81736-5. PMID 9753324. S2CID 12465062.
- ^ Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, et al. (June 2008). "De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy". Nature Genetics. 40 (6): 782–788. doi:10.1038/ng.150. PMID 18469812. S2CID 1113528.
- ^ Mori T, Kasem EA, Suzuki-Kouyama E, Cao X, Li X, Kurihara T, et al. (July 2019). "Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B". Molecular Psychiatry. 24 (7): 1079–1092. doi:10.1038/s41380-018-0338-4. PMC 6756202. PMID 30610199.
- ^ a b Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, et al. (May 2004). "Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins". The Journal of Biological Chemistry. 279 (21): 22331–22346. doi:10.1074/jbc.M400285200. PMID 15024025.
- ^ a b c d Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA (April 2004). "A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels". The Journal of Biological Chemistry. 279 (18): 19051–19063. doi:10.1074/jbc.M400284200. PMID 14960569.
- ^ a b Borg JP, Straight SW, Kaech SM, de Taddéo-Borg M, Kroon DE, Karnak D, et al. (November 1998). "Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting". The Journal of Biological Chemistry. 273 (48): 31633–31636. doi:10.1074/jbc.273.48.31633. PMID 9822620.
- ^ Borg JP, Lõpez-Figueroa MO, de Taddèo-Borg M, Kroon DE, Turner RS, Watson SJ, Margolis B (February 1999). "Molecular analysis of the X11-mLin-2/CASK complex in brain". The Journal of Neuroscience. 19 (4): 1307–1316. doi:10.1523/JNEUROSCI.19-04-01307.1999. PMC 6786035. PMID 9952408.
- ^ Schuh K, Uldrijan S, Gambaryan S, Roethlein N, Neyses L (March 2003). "Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK". The Journal of Biological Chemistry. 278 (11): 9778–9783. doi:10.1074/jbc.M212507200. PMID 12511555.
- ^ Wang GS, Hong CJ, Yen TY, Huang HY, Ou Y, Huang TN, et al. (April 2004). "Transcriptional modification by a CASK-interacting nucleosome assembly protein". Neuron. 42 (1): 113–128. doi:10.1016/S0896-6273(04)00139-4. PMID 15066269.
- ^ a b Chetkovich DM, Bunn RC, Kuo SH, Kawasaki Y, Kohwi M, Bredt DS (August 2002). "Postsynaptic targeting of alternative postsynaptic density-95 isoforms by distinct mechanisms". The Journal of Neuroscience. 22 (15): 6415–6425. doi:10.1523/JNEUROSCI.22-15-06415.2002. PMC 6758133. PMID 12151521.
- ^ Nix SL, Chishti AH, Anderson JM, Walther Z (December 2000). "hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions". The Journal of Biological Chemistry. 275 (52): 41192–41200. doi:10.1074/jbc.M002078200. PMID 10993877.
- ^ Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G (March 2001). "Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells". The Journal of Biological Chemistry. 276 (12): 9291–9296. doi:10.1074/jbc.M006991200. PMID 11120739.
- ^ Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D (September 2000). "Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1". The Journal of Biological Chemistry. 275 (36): 27979–27988. doi:10.1074/jbc.M002363200. PMID 10856295.
- ^ Qi J, Su Y, Sun R, Zhang F, Luo X, Yang Z, Luo X (March 2005). "CASK inhibits ECV304 cell growth and interacts with Id1". Biochemical and Biophysical Research Communications. 328 (2): 517–521. doi:10.1016/j.bbrc.2005.01.014. PMID 15694377.
- ^ Lehtonen S, Lehtonen E, Kudlicka K, Holthöfer H, Farquhar MG (September 2004). "Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin". The American Journal of Pathology. 165 (3): 923–936. doi:10.1016/S0002-9440(10)63354-8. PMC 1618613. PMID 15331416.
- ^ Fallon L, Moreau F, Croft BG, Labib N, Gu WJ, Fon EA (January 2002). "Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain". The Journal of Biological Chemistry. 277 (1): 486–491. doi:10.1074/jbc.M109806200. PMID 11679592.
- ^ a b Zhang Y, Luan Z, Liu A, Hu G (May 2001). "The scaffolding protein CASK mediates the interaction between rabphilin3a and beta-neurexins". FEBS Letters. 497 (2–3): 99–102. doi:10.1016/S0014-5793(01)02450-4. PMID 11377421. S2CID 33119468.
- ^ Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM, Wood DF (July 1998). "Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells". The Journal of Cell Biology. 142 (1): 129–138. doi:10.1083/jcb.142.1.129. PMC 2133028. PMID 9660868.
Further reading
[edit]- Zhu ZQ, Wang D, Xiang D, Yuan YX, Wang Y (January 2014). "Calcium/calmodulin-dependent serine protein kinase is involved in exendin-4-induced insulin secretion in INS-1 cells". Metabolism. 63 (1): 120–126. doi:10.1016/j.metabol.2013.09.009. PMID 24140090.
- Wang Y, Li R, Du D, Zhang C, Yuan H, Zeng R, Chen Z (April 2006). "Proteomic analysis reveals novel molecules involved in insulin signaling pathway". Journal of Proteome Research. 5 (4): 846–855. CiteSeerX 10.1.1.583.5128. doi:10.1021/pr050391m. PMID 16602692.
- Mukherjee K, Slawson JB, Christmann BL, Griffith LC (2014). "Neuron-specific protein interactions of Drosophila CASK-β are revealed by mass spectrometry". Frontiers in Molecular Neuroscience. 7: 58. doi:10.3389/fnmol.2014.00058. PMC 4075472. PMID 25071438.
- Wei JL, Fu ZX, Fang M, Zhou QY, Zhao QN, Guo JB, et al. (September 2014). "High expression of CASK correlates with progression and poor prognosis of colorectal cancer". Tumour Biology. 35 (9): 9185–9194. doi:10.1007/s13277-014-2179-3. PMID 24927672. S2CID 1809280.
- Hata Y, Butz S, Südhof TC (April 1996). "CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins". The Journal of Neuroscience. 16 (8): 2488–2494. doi:10.1523/JNEUROSCI.16-08-02488.1996. PMC 6578772. PMID 8786425.
- Daniels DL, Cohen AR, Anderson JM, Brünger AT (April 1998). "Crystal structure of the hCASK PDZ domain reveals the structural basis of class II PDZ domain target recognition". Nature Structural Biology. 5 (4): 317–325. doi:10.1038/nsb0498-317. PMID 9546224. S2CID 20608889.
- Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M (July 1998). "Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses". The Journal of Cell Biology. 142 (1): 139–151. doi:10.1083/jcb.142.1.139. PMC 2133027. PMID 9660869.
- Butz S, Okamoto M, Südhof TC (September 1998). "A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain". Cell. 94 (6): 773–782. doi:10.1016/S0092-8674(00)81736-5. PMID 9753324.
- Borg JP, Straight SW, Kaech SM, de Taddéo-Borg M, Kroon DE, Karnak D, et al. (November 1998). "Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting". The Journal of Biological Chemistry. 273 (48): 31633–31636. doi:10.1074/jbc.273.48.31633. PMID 9822620.
- Borg JP, Lõpez-Figueroa MO, de Taddèo-Borg M, Kroon DE, Turner RS, Watson SJ, Margolis B (February 1999). "Molecular analysis of the X11-mLin-2/CASK complex in brain". The Journal of Neuroscience. 19 (4): 1307–1316. doi:10.1523/JNEUROSCI.19-04-01307.1999. PMC 6786035. PMID 9952408.
- Maximov A, Südhof TC, Bezprozvanny I (August 1999). "Association of neuronal calcium channels with modular adaptor proteins". The Journal of Biological Chemistry. 274 (35): 24453–24456. doi:10.1074/jbc.274.35.24453. PMID 10455105.
- Hsueh YP, Sheng M (September 1999). "Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development". The Journal of Neuroscience. 19 (17): 7415–7425. doi:10.1523/JNEUROSCI.19-17-07415.1999. PMC 6782500. PMID 10460248.
- Hsueh YP, Wang TF, Yang FC, Sheng M (March 2000). "Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2". Nature. 404 (6775): 298–302. Bibcode:2000Natur.404..298H. doi:10.1038/35005118. PMID 10749215. S2CID 4415747.
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D (September 2000). "Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1". The Journal of Biological Chemistry. 275 (36): 27979–27988. doi:10.1074/jbc.M002363200. PMID 10856295.
- Nix SL, Chishti AH, Anderson JM, Walther Z (December 2000). "hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions". The Journal of Biological Chemistry. 275 (52): 41192–41200. doi:10.1074/jbc.M002078200. PMID 10993877.
- Stevenson D, Laverty HG, Wenwieser S, Douglas M, Wilson JB (October 2000). "Mapping and expression analysis of the human CASK gene". Mammalian Genome. 11 (10): 934–937. doi:10.1007/s003350010170. PMID 11003712. S2CID 35231493.
- Biederer T, Südhof TC (December 2000). "Mints as adaptors. Direct binding to neurexins and recruitment of munc18". The Journal of Biological Chemistry. 275 (51): 39803–39806. doi:10.1074/jbc.C000656200. PMID 11036064.
- Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G (March 2001). "Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells". The Journal of Biological Chemistry. 276 (12): 9291–9296. doi:10.1074/jbc.M006991200. PMID 11120739.
- Hsueh YP, Roberts AM, Volta M, Sheng M, Roberts RG (June 2001). "Bipartite interaction between neurofibromatosis type I protein (neurofibromin) and syndecan transmembrane heparan sulfate proteoglycans". The Journal of Neuroscience. 21 (11): 3764–3770. doi:10.1523/JNEUROSCI.21-11-03764.2001. PMC 6762697. PMID 11356864.
- Zhang Y, Luan Z, Liu A, Hu G (May 2001). "The scaffolding protein CASK mediates the interaction between rabphilin3a and beta-neurexins". FEBS Letters. 497 (2–3): 99–102. doi:10.1016/S0014-5793(01)02450-4. PMID 11377421. S2CID 33119468.
- Fallon L, Moreau F, Croft BG, Labib N, Gu WJ, Fon EA (January 2002). "Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain". The Journal of Biological Chemistry. 277 (1): 486–491. doi:10.1074/jbc.M109806200. PMID 11679592.
- Olsen O, Liu H, Wade JB, Merot J, Welling PA (January 2002). "Basolateral membrane expression of the Kir 2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex". American Journal of Physiology. Cell Physiology. 282 (1): C183 – C195. doi:10.1152/ajpcell.00249.2001. PMID 11742811.
External links
[edit]- Human CASK genome location and CASK gene details page in the UCSC Genome Browser.


 French
French Deutsch
Deutsch