Dysprosium(III) iodide
 | |
| Names | |
|---|---|
| Other names Dysprosium triiodide, triiododysprosium | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.035.888 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| DyI3 | |
| Molar mass | 543.213 g·mol−1 |
| Appearance | yellow-green flaky solid |
| Density | g/cm3 |
| Melting point | 955 °C (1,751 °F; 1,228 K) |
| Boiling point | 1,320 °C (2,410 °F; 1,590 K) |
| soluble | |
| Structure | |
| trigonal | |
| Related compounds | |
Related compounds | Ytterbium(III) iodide |
| Hazards | |
| GHS labelling: | |
 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dysprosium(III) iodide is a binary inorganic compound of dysprosium and iodine with the chemical formula DyI
3.[1]
Synthesis
[edit]Dysprosium(III) iodide can be obtained by reacting dysprosium with iodine.
- 2Dy + 3I → 2DyI3
Dysprosium(III) iodide can be obtained using the effect of mercury diiodide on dysprosium filings:
- 2Dy + 3HgI2 → 2DyI3 + 3Hg
Physical properties
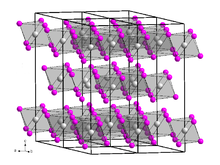
[edit]Dysprosium(III) iodide is a hygroscopic yellow-green flaky solid[2] that is soluble in water.[3] The compound has a trigonal crystal structure of the bismuth(III) iodide type with the space group R3.
Uses
[edit]Dysprosium(III) iodide is used in gas discharge lamps to generate white light.[4]
References
[edit]- ^ "dysprosium triiodide". NIST. Retrieved 5 April 2023.
- ^ Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 3117. ISBN 978-0-412-30120-9. Retrieved 5 April 2023.
- ^ "Dysprosium(III) iodide, ultra dry, 99.99% (REO), Thermo Scientific Chemicals". fishersci.com. Retrieved 5 April 2023.
- ^ Emsley, John (2003). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press. p. 131. ISBN 978-0-19-850340-8. Retrieved 5 April 2023.


 French
French Deutsch
Deutsch