List of opioids
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (January 2012) |

This is a list of opioids, opioid antagonists and inverse agonists.
Opium and poppy straw derivatives
[edit]
Crude opiate extracts whole opium products
[edit]Natural opiates
[edit]Opium alkaloids
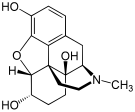
[edit]Structures
| Morphides | ||||
|---|---|---|---|---|
 Codeine Codeine |  Morphine Morphine |  Oripavine Oripavine |  Pseudomorphine Pseudomorphine |  Thebaine Thebaine |
Alkaloid salts mixtures
[edit]- Pantopon
- Papaveretum (Omnopon)
- Tetrapon
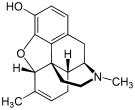
Semisynthetics including Bentley compounds
[edit]Morphine family
[edit]- 14-Hydroxymorphine [1]
- 2,4-Dinitrophenylmorphine
- 6-Methyldihydromorphine [2]
- 6-Methylenedihydrodesoxymorphine
- 6-Acetyldihydromorphine [3]
- Azidomorphine
- Chlornaltrexamine
- Chloroxymorphamine [4]
- Desomorphine (dihydrodesoxymorphine)
- Dihydromorphine
- Ethyldihydromorphine [5][full citation needed]
- Hydromorphinol
- Methyldesorphine
- Morphine methylbromide
- N-Phenethylnordesomorphine
- N-Phenethyl-14-ethoxymetopon
- N-Phenethylnormorphine
- 6-Nicotinoyldihydromorphine (metabolite of nicodicodeine)
- RAM-378
- Ro-1539
Structures of Morphine family
3,6-diesters of morphine
[edit]- Acetylpropionylmorphine
- 3,6-Dibutanoylmorphine
- Diacetyldihydromorphine (dihydroheroin, acetylmorphinol)
- Dibutyrylmorphine [1]
- Dibenzoylmorphine (first designer drug)
- Diformylmorphine
- Dipropanoylmorphine
- Heroin (diacetylmorphine)
- Nicomorphine
Structures
| 3,6-diesters of morphine | ||||
|---|---|---|---|---|
 Acetylpropionylmorphine Acetylpropionylmorphine | 3,6-Dibutanoylmorphine |  Diacetyldihydromorphine Diacetyldihydromorphine(dihydroheroin, acetylmorphinol) | ||
 Dibutyrylmorphine Dibutyrylmorphine |  Dibenzoylmorphine Dibenzoylmorphine |  Diformylmorphine Diformylmorphine | ||
 Dipropanoylmorphine Dipropanoylmorphine |  Heroin Heroin(diacetylmorphine) |  Nicomorphine Nicomorphine | ||
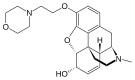
Codeine-dionine family
[edit]- 6-Monoacetylcodeine
- Benzylmorphine
- Codeine methylbromide
- Desocodeine [2] [permanent dead link]
- Dimethylmorphine (6-O-Methylcodeine)
- Ethyldihydromorphine [3] [permanent dead link]
- Methyldihydromorphine (dihydroheterocodeine)
- Ethylmorphine (dionine)
- Heterocodeine
- Isocodeine [4]
- Pholcodine (morpholinylethylmorphine)
- Myrophine
- Thebacon
- Transisocodeine [5]
Structures
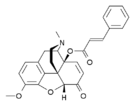
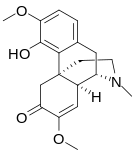
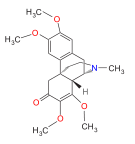
Morphinones and morphols
[edit]- 14-Cinnamoyloxycodeinone
- 14-Ethoxymetopon
- 14-Methoxymetopon
- 14-Phenylpropoxymetopon
- 3-Acetyloxymorphone
- 3,14-Diacetyloxymorphone
- 7-Spiroindanyloxymorphone
- 8,14-Dihydroxydihydromorphinone [6]
- Acetylcodone
- Acetylmorphone
- α-hydrocodol (=dihydrocodeine, )
- Benzhydrocodone
- Bromoisopropropyldihydromorphinone cas?
- Codeinone
- Codoxime
- Conorfone (codorphone)
- IBNtxA
- Thebacon (acetyldihydrocodeinone, dihydrocodeinone enol acetate)
- Hydrocodone
- Hydromorphone
- Hydroxycodeine [7]
- Metopon (=methyldihydromorphinone)
- Morphenol [8]
- Morphinone
- Morphol [9]
- N-Phenethyl-14-ethoxymetopon
- Noroxymorphone
- Oxycodone
- Oxymorphol
- Oxymorphone
- Pentamorphone
- Semorphone
Structures
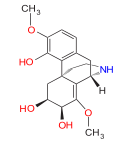
Morphides
[edit]- α-Chlorocodide (= chlorocodide) [10]
- β-Chlorocodide [11]
- α-Chloromorphide (= chloromorphide)
- Bromocodide [12]
- Bromomorphide [13]
- Chlorodihydrocodide [14]
- Chloromorphide
- Codide [15]
Structures
Dihydrocodeine series
[edit]Structures
Nitrogen morphine derivatives
[edit]Structures
| Morphides | ||||
|---|---|---|---|---|
 1-Nitrocodeine 1-Nitrocodeine |  Codeine-N-oxide Codeine-N-oxide |  Morphine-N-oxide Morphine-N-oxide | ||
Hydrazones
[edit]Structures
| Hydrazones | ||||
|---|---|---|---|---|
 Oxymorphazone Oxymorphazone | ||||
Halogenated morphine derivatives
[edit]Active opiate metabolites
[edit]Structures
| Active opiate metabolites | ||||
|---|---|---|---|---|
 Codeine-N-oxide Codeine-N-oxide |  Heroin-7,8-oxide Heroin-7,8-oxide |  Morphine-6-glucuronide Morphine-6-glucuronide | 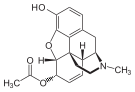 6-Monoacetylmorphine 6-Monoacetylmorphine | |
 Morphine-N-oxide Morphine-N-oxide |  Naltrexol Naltrexol |  Norcodeine Norcodeine |  Normorphine Normorphine | |
Morphinans
[edit]
Morphinan series
[edit]- 3-Hydroxymorphinan
- 4-Chlorophenylpyridomorphinan [21]
- Cyclorphan
- Levargorphan [22]
- Levorphanol
- Levophenacylmorphan
- Levomethorphan
- Methorphan (racemethorphan)
- Morphanol (racemorphanol)
- Norlevorphanol
- N-Methylmorphinan [23]
- Oxilorphan
- Phenomorphan
- Proxorphan
- Ro4-1539
- Stephodeline [24] [permanent dead link]Xorphanol
Structures
Others
[edit]Structures
Benzomorphans
[edit]- 5,9 alpha-diethyl-2-hydroxybenzomorphan (5,9-DEHB) [25]
- 8-Carboxamidocyclazocine (8-CAC)
- Alazocine
- Anazocine [26]
- Bremazocine
- Butinazocine [27]
- Carbazocine [28]
- Cogazocine
- Cyclazocine
- Dezocine
- Eptazocine
- Etazocine [29] [permanent dead link]
- Ethylketazocine [30]
- Fedotozine
- Fluorophen [31]
- Gemazocine [32] [permanent dead link]
- Ibazocine [33] [permanent dead link]
- Ketazocine
- Metazocine
- Moxazocine [34]
- Pentazocine
- Phenazocine
- Quadazocine [35]
- SKF-10047
- Tonazocine
- Volazocine
- Zenazocine[36]
Structures
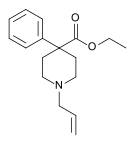
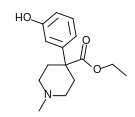
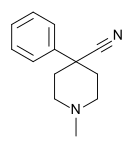
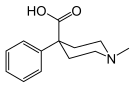
4-Phenylpiperidines
[edit]
Pethidines (meperidines)
[edit]- 4-Fluoropethidine
- Allylnorpethidine
- Anileridine
- Benzethidine
- Carperidine
- Difenoxin
- Diphenoxylate
- Etoxeridine (carbetidine)
- Furethidine
- Hydroxypethidine (bemidone)
- Morpheridine
- Meperidine-N-oxide
- Oxpheneridine (carbamethidine)
- Pethidine (meperidine)
- Pethidine intermediate A
- Pethidine intermediate B (norpethidine)
- Pethidine intermediate C (pethidinic acid)
- Pheneridine
- Phenoperidine
- Piminodine
- Properidine (ipropethidine)
- Sameridine
Structures
Prodines
[edit]- Allylprodine
- (α/β)-Meprodine
- Desmethylprodine (MPPP)
- PEPAP
- (α/β)-Prodine
- Prosidol
- Trimeperidine (promedol)
Structures
Ketobemidones
[edit]Structures
Others
[edit]Structures
| Other phenylpiperidines | |||
|---|---|---|---|
 Alvimopan Alvimopan |  Loperamide Loperamide |  LS-115509 LS-115509 |  Picenadol Picenadol |
Open chain opioids
[edit]
Amidones
[edit]Structures
Methadols
[edit]Moramides
[edit]Thiambutenes
[edit]Structures
Phenalkoxams
[edit]Structures
| Phenalkoxams | ||||
|---|---|---|---|---|
 Dextropropoxyphene |  | 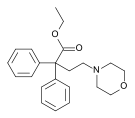 |  |  |
Ampromides
[edit]Others
[edit]Anilidopiperidines
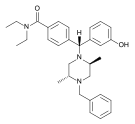
[edit]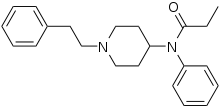
- 3-Allylfentanyl
- 3-Methylfentanyl
- 3-Methylthiofentanyl
- 4-Phenylfentanyl
- Alfentanil
- α-Methylacetylfentanyl
- α-Methylfentanyl
- α-Methylthiofentanyl
- Benzylfentanyl [40]
- β-hydroxyfentanyl
- β-hydroxythiofentanyl
- β-Methylfentanyl
- Brifentanil
- Butyrfentanyl
- Carfentanil
- Fentanyl
- Lofentanil
- N-Methylcarfentanil
- Mirfentanil
- Ocfentanil
- Ohmefentanyl
- Parafluorofentanyl
- Phenaridine
- R-30490
- Remifentanil
- Sufentanil
- Thenylfentanyl [41]
- Thiofentanyl
- Trefentanil
Structures
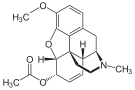
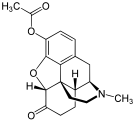
Oripavine derivatives
[edit]
- 7-PET
- Acetorphine
- Alletorphine (N-allyl-noretorphine)
- BU-48
- Buprenorphine
- Buprenorphine-3-glucuronide
- Cyprenorphine
- Dihydroetorphine
- Etorphine
- Homprenorphine
- 18,19-Dehydrobuprenorphine (HS-599) [42] [43]
- N-cyclopropylmethylnoretorphine [44]
- Nepenthone [45] [46]
- Norbuprenorphine
- Norbuprenorphine-3-glucuronide
- Thevinone [47]
- Thienorphine [48]
Structures
Phenazepanes
[edit]Pirinitramides
[edit]Benzimidazoles
[edit]Indoles
[edit]Structures
Beta-Amino Ketones
[edit]Structures
Diphenylmethylpiperazines
[edit]Opioid peptides
[edit]
Dynorphins
[edit]Structures
| Dynorphins | ||||
|---|---|---|---|---|
 Big dynorphin Big dynorphin |  Dynorphin A Dynorphin A |  Dynorphin B Dynorphin B | ||
Endomorphins
[edit]Endorphins
[edit]Structures
Enkephalins
[edit]Structures
| Enkephalins | ||||
|---|---|---|---|---|
 DAMGO DAMGO | ||||
Propeptides
[edit]Others / unknown
[edit]- Adrenorphin
- Amidorphin
- Biphalin
- Casokefamide
- Casomorphins
- Cytochrophin-4
- DALDA (Tyr-D-Arg-Phe-Lys-NH2)[49]
- Deltorphin I
- Deltorphin II
- Deprolorphin
- Dermorphin
- DPDPE
- Frakefamide
- Gliadorphin
- Gluten exorphins
- Hemorphin-4
- Metkefamide
- Morphiceptin
- Nociceptin
- Octreotide
- Opiorphin
- Rubiscolin
- Soymorphins
- Spinorphin
- TRIMU 5
- Tynorphin
- Valorphin
- Zyklophin
Structures
| Other or unknown opioid peptides | ||||
|---|---|---|---|---|
 Adrenorphin Adrenorphin |  Amidorphin Amidorphin | 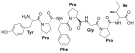 Casomorphin Casomorphin |  DALDA DALDA | |
 DPDPE DPDPE | 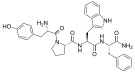 Endomorphin-1 Endomorphin-1 Endomorphin-2 Endomorphin-2 |  Gliadorphin Gliadorphin | 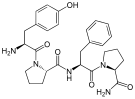 Morphiceptin Morphiceptin | |
 Nociceptin Nociceptin |  Octreotide Octreotide |  Opiorphin Opiorphin |  Rubiscolin Rubiscolin |  TRIMU 5 TRIMU 5 |
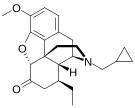
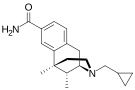
Others
[edit]- 3-(3-Methoxyphenyl)-3-ethoxycarbonyltropane
- AD-1211
- AH-7921
- Axomadol
- Azaprocin
- BDPC
- Bisnortilidine [50] [51]
- BRL-52537
- Bromadol
- Bromadoline
- Ciprefadol
- Ciramadol
- Doxpicomine
- Enadoline
- Faxeladol
- GR-89696
- Herkinorin
- ICI-199,441
- ICI-204,448
- Ketamine
- KNT-42
- LPK-26
- Lufuradom
- Metofoline
- MT-45
- Desmethylclozapine
- Nexeridine
- NNC 63-0532
- Nortilidine
- O-Desmethyltramadol
- Phenadone
- Phencyclidine
- Prodilidine
- Profadol
- Ro64-6198
- Salvinorin A
- Salvinorin B ethoxymethyl ether
- Salvinorin B methoxymethyl ether
- SB-612,111
- SC-17599
- RWJ-394,674
- TAN-67
- Tapentadol
- Thiobromadol (C-8813)
- Tifluadom
- Tilidine
- Tramadol
- Trimebutine
- U-47700
- U-50,488
- U-69,593
- Viminol
- 1-(4-Nitrophenylethyl)piperidylidene-2-(4-chlorophenyl)sulfonamide (W-18)
Opioid antagonists and inverse agonists
[edit]- 4-Caffeoyl-1,5-quinide
- 5'-Guanidinonaltrindole
- β-Funaltrexamine
- 6β-Naltrexol
- 6β-Naltrexol-d4
- Alvimopan
- AT-076
- Binaltorphimine
- BU09059
- Buprenorphine
- Chlornaltrexamine
- Clocinnamox
- Cyclazocine
- Cyprodime
- Diacetylnalorphine [52]
- Diprenorphine (M5050)
- Fedotozine
- ICI-174864
- J-113,397
- JDTic
- JTC-801
- Levallorphan
- LY-2456302
- LY-255582
- Methocinnamox
- Methylnaltrexone
- ML350
- Naldemedine
- Nalfurafine
- Nalmefene
- Nalmexone [53]
- Nalodeine (N-allylnorcodeine) [54]
- Naloxazone
- Naloxegol
- Naloxol
- Naloxonazine
- Naloxone
- Naloxone benzoylhydrazone
- Nalorphine
- Naltrexone
- Naltriben
- Naltrindole
- Norbinaltorphimine
- Oxilorphan
- PF-4455242
- S-allyl-3-hydroxy-17-thioniamorphinan (SAHTM)[55]
- Samidorphan
- SR-16430
Biased ligands
[edit]Receptor heteromer targeting ligands
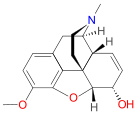
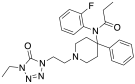
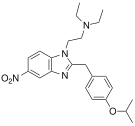
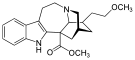
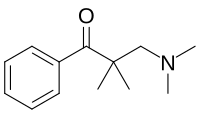
[edit]Uncategorized opioids
[edit]Structures
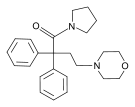
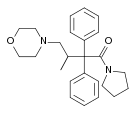
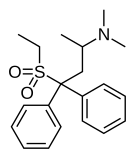
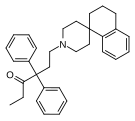
| Uncategorized opioids | ||||
|---|---|---|---|---|
 |  |  | ||
 |  |  | ||
 | 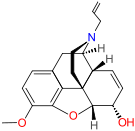 Nalodeine Nalodeine |  |  | |
 | ||||
Combination drug formulations containing opioids
[edit]- Buprenorphine/naloxone
- Buprenorphine/samidorphan (ALKS-5461)
- Co-codamol (codeine phosphate/paracetamol)
- Co-codaprin (codeine phosphate/aspirin)
- Co-dydramol (dihydrocodeine tartrate/paracetamol)
- Co-proxamol (dextropropoxyphene/paracetamol)
- Fentanyl/fluanisone
- Hydrocodone/ibuprofen
- Hydrocodone/paracetamol
- Loperamide/simethicone
- Morphine/naltrexone
- Naltrexone/bupropion
- Oxycodone/aspirin
- Oxycodone/naloxone
- Oxycodone/paracetamol
See also
[edit]- List of opioids by visual 2D molecular skeletal renderings (bundled remotely, click "show" after following link)
- List of Schedule I drugs (US)
- Gray death
References
[edit]- ^ ChemIndex CAS Registry Number 3371-56-0
- ^ ChemIndex CAS Registry Number 509-56-8
- ^ ChemIndex CAS Registry Number 63715-94-6
- ^ NCBI PubChem SID 750205
- ^ chembase [permanent dead link] cbid_346222
- ^ Gomes I, Fujita W, Gupta A, Saldanha SA, Saldanha AS, Negri A, Pinello CE, Eberhart C, Roberts E, Filizola M, Hodder P, Devi LA (2013). "Identification of a μ-δ opioid receptor heteromer-biased agonist with antinociceptive activity". Proc. Natl. Acad. Sci. U.S.A. 110 (29): 12072–7. Bibcode:2013PNAS..11012072G. doi:10.1073/pnas.1222044110. PMC 3718106. PMID 23818586.
- ^ Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese PS (2011). "N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of μ/kappa-opioid heteromers". Proc. Natl. Acad. Sci. U.S.A. 108 (12): 5098–103. Bibcode:2011PNAS..108.5098Y. doi:10.1073/pnas.1016277108. PMC 3064379. PMID 21385944.
External links
[edit]- Carbonate derivatives of 14β-hydroxycodeine "viz., 14β-hydroxy-6-O-(methoxycarbonyl)codeine, 6-O-methoxycarbonyl-14β-(methoxycarbonyloxy)codeine, and 14β-acetoxy-6-O-methoxy-carbonylcodeine, potential substrates for ring C modification in morphinane (sic) alkaloids, were synthesized for the first time." Russian Chemical Bulletin. August 2008, Volume 57, Issue 8, pp 1773–1774. Date: 11 Aug 2009; I. V. Evsikova, S. K. Moiseev, P. V. Petrovskii, V. N. Kalinin. Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1739–1740


 French
French Deutsch
Deutsch