Promestriene
 | |
| Clinical data | |
|---|---|
| Trade names | Colpotrofin, Colpotrophine, Delipoderm |
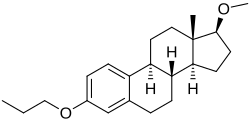
| Other names | Estradiol 3-propyl 17β-methyl diether; 17β-Methoxy-3-propoxyestra-1,3,5(10)-triene |
| Routes of administration | Topical |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.049.401 |
| Chemical and physical data | |
| Formula | C22H32O2 |
| Molar mass | 328.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Promestriene (INN) (brand names Colpotrofin, Colpotrophine, Delipoderm), also known as estradiol 3-propyl 17β-methyl diether, is a synthetic estrogen which is used topically in a 1% cream formulation for the treatment of vaginal atrophy in women.[1][2][3][4][5] It is the 3-propyl and 17β-methyl diether of estradiol and does not appear to convert into estradiol in the body.[1][6] Promestriene is minimally absorbed and appears to have negligible systemic estrogenic effect.[1] The drug has been described as a tropic agent and antiseborrheic.[2] It has not been found to be effective in the treatment of pattern hair loss or other conditions of cutaneous androgenization.[7][8] Promestriene was first introduced in France in 1974 and has been marketed in 34 countries worldwide.[1] It has been used in millions of women.[1]
See also
[edit]References
[edit]- ^ a b c d e Del Pup L, Di Francia R, Cavaliere C, Facchini G, Giorda G, De Paoli P, Berretta M (November 2013). "Promestriene, a specific topic estrogen. Review of 40 years of vaginal atrophy treatment: is it safe even in cancer patients?". Anti-Cancer Drugs. 24 (10): 989–998. doi:10.1097/CAD.0b013e328365288e. PMID 24080714. S2CID 3458356.
- ^ a b Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1671–. ISBN 978-0-412-46630-4.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1248, 1266, 1318, 1557. ISBN 978-3-88763-075-1.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 270–271. ISBN 978-0-8155-1856-3.
- ^ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 289, 33 2. ISBN 978-3-7692-2114-5.
- ^ Santos I, Clissold S (September 2010). "Urogenital disorders associated with oestrogen deficiency: the role of promestriene as topical oestrogen therapy". Gynecological Endocrinology. 26 (9): 644–651. doi:10.3109/09513591003767948. PMID 20374067. S2CID 243338.
- ^ Orfanos CE, Montagna W, Stüttgen G (6 December 2012). Hair Research: Status and Future Aspects; Proceedings of the First International Congress on Hair Research, Hamburg, March 13th–16, 1979. Springer Science & Business Media. pp. 553–. ISBN 978-3-642-81650-5.
- ^ Beylot C (1 October 1998). "Menopause, Skin, and Cosmetology". In Baran R, Maibach HI (eds.). Textbook of Cosmetic Dermatology. CRC Press. pp. 493–. ISBN 978-1-85317-478-0.


 French
French Deutsch
Deutsch