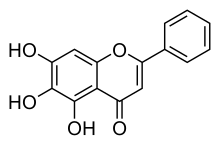
Baicalein
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Biacalein; Noroxylin |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.911 |
| Chemical and physical data | |
| Formula | C15H10O5 |
| Molar mass | 270.240 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid,[1] originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also a constituent of Oroxylum indicum (Indian trumpetflower) and thyme.[2] It is the aglycone of baicalin.
Pharmacology
[edit]Baicalein, along with its glucuronide baicalin, is a positive allosteric modulator of the benzodiazepine site and a non-benzodiazepine site of the GABAA receptor, but with an affinity over 250× lower than diazepam.[3][4][5] It displays subtype selectivity for α2 and α3 subunit-containing GABAA receptors.[6]
The flavonoid has been shown to inhibit certain types of lipoxygenases.[7]
Baicalein is an inhibitor of CYP2C9,[8] an enzyme of the cytochrome P450 system that metabolizes drugs in the body.
A derivative of baicalin is a known prolyl endopeptidase inhibitor.[9]
See also
[edit]References
[edit]- ^ "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2024. Retrieved 9 May 2024.
- ^ Matsumoto T (2008). Phytochemistry Research Progress. Nova Publishers. ISBN 9781604562323.
- ^ Zhang SQ, Obregon D, Ehrhart J, Deng J, Tian J, Hou H, et al. (September 2013). "Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model". Journal of Neuroscience Research. 91 (9): 1239–1246. doi:10.1002/jnr.23244. PMC 3810722. PMID 23686791.
- ^ Liao JF, Wang HH, Chen MC, Chen CC, Chen CF (August 1998). "Benzodiazepine binding site-interactive flavones from Scutellaria baicalensis root". Planta Medica. 64 (6): 571–572. doi:10.1055/s-2006-957517. PMID 9776664. S2CID 260251315.
- ^ Roberts AA (2004). "Testing efficacy of natural anxiolytic compounds". In Cooper EL, Yamaguchi N (eds.). Complementary and Alternative Approaches to Biomedicine. Advances in Experimental Medicine and Biology. Vol. 546. pp. 181–191. doi:10.1007/978-1-4757-4820-8_13. ISBN 978-1-4419-3441-3. PMID 15584374.
- ^ Wang F, Xu Z, Ren L, Tsang SY, Xue H (December 2008). "GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin". Neuropharmacology. 55 (7): 1231–1237. doi:10.1016/j.neuropharm.2008.07.040. PMID 18723037. S2CID 20133964.
- ^ Deschamps JD, Kenyon VA, Holman TR (June 2006). "Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases". Bioorganic & Medicinal Chemistry. 14 (12): 4295–4301. doi:10.1016/j.bmc.2006.01.057. PMID 16500106. S2CID 645610.
- ^ Si D, Wang Y, Zhou YH, Guo Y, Wang J, Zhou H, et al. (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols" (PDF). Drug Metabolism and Disposition. 37 (3): 629–634. doi:10.1124/dmd.108.023416. PMID 19074529. S2CID 285706. Archived from the original (PDF) on 2008-12-17. Retrieved 2009-02-19.
- ^ Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E (August 2008). "Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor". Bioorganic & Medicinal Chemistry. 16 (15): 7516–7524. doi:10.1016/j.bmc.2008.04.067. PMID 18650094.


 French
French Deutsch
Deutsch