Substituted benzofuran

The substituted benzofurans are a class of chemical compounds based on the heterocyclic and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold,[1][2][3] but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached (at any position), and combined with a range of other substituents.[4][5][6][7] Some psychoactive derivatives from this family have been sold under the name Benzofury.[8]
List of substituted benzofurans
[edit]The derivatives may be produced by substitutions at six locations of the benzofuran molecule, as well as saturation of the 2,3- double bond.
The following table displays notable derivatives that have been reported:[9][10][11][12][13][14][15][16][17][18][19]
| Structure | Compound | CAS # | R2 | R3 | R4 | R5 | R6 | R7 | Other |
|---|---|---|---|---|---|---|---|---|---|
 | 2-APB | 30455-73-3 | 2-aminopropyl | H | H | H | H | H | - |
 | 2-MAPB | 806596-15-6 | 2-(methylamino)propyl | H | H | H | H | H | - |
 | 2-EAPB | 2-(ethylamino)propyl | H | H | H | H | H | - | |
 | BPAP | 260550-89-8 | 2-(propylamino)pentyl | H | H | H | H | H | - |
 | Brofaromine | 63638-91-5 | 4-piperidinyl | H | H | methoxy | H | bromo | - |
 | 3-APB | 105909-13-5 | H | 2-aminopropyl | H | H | H | H | - |
 | Dimemebfe | 140853-58-3 | H | 2-(dimethyl-amino)ethyl | H | methoxy | H | H | - |
 | Mebfap | 140853-59-4 | H | 2-aminopropyl | H | methoxy | H | H | - |
 | 5-MeO-DiBF | H | 2-(diisopropyl-amino)ethyl | H | methoxy | H | H | - | |
 | 4-APB | 286834-82-0 | H | H | 2-aminopropyl | H | H | H | - |
 | DOB-5-HEMIFLY (5-MeO-7-Br-4-APDB) | H | H | 2-aminopropyl | methoxy | H | bromo | 2,3-dihydro | |
 | 5-APB | 286834-81-9 | H | H | H | 2-aminopropyl | H | H | - |
 | 5-MAPB | 1354631-77-8 | H | H | H | 2-(methylamino)propyl | H | H | - |
| 5-EAPB | 1445566-01-7 | H | H | H | 2-(ethylamino)propyl | H | H | - | |
 | 5-APB-NBOMe | H | H | H | 2-[(2-methoxybenzyl)-amino]propyl | H | H | - | |
| 6-APB | 286834-85-3 | H | H | H | H | 2-aminopropyl | H | - | |
 | 6-MAPB | 1354631-79-0 | H | H | H | H | 2-(methylamino)propyl | H | - |
| 6-EAPB | 1632539-47-9 | H | H | H | H | 2-(ethylamino)propyl | H | - | |
 | 5-AEDB | H | H | H | 2-aminoethyl | H | H | 2,3-dihydro | |
| 5-APDB | 152624-03-8 | H | H | H | 2-aminopropyl | H | H | 2,3-dihydro | |
 | 5-MAPDB | 1354631-78-9 | H | H | H | 2-(methylamino)propyl | H | H | 2,3-dihydro |
 | 5-EAPDB | H | H | H | 2-(ethylamino)propyl | H | H | 2,3-dihydro | |
| 6-APDB | 1354631-78-9 | H | H | H | H | 2-aminopropyl | H | 2,3-dihydro | |
 | 6-MAPDB | 1354631-81-4 | H | H | H | H | 2-(methylamino)propyl | H | 2,3-dihydro |
 | 6-EAPDB | H | H | H | H | 2-(ethylamino)propyl | H | 2,3-dihydro | |
 | bk-5-MAPB | H | H | H | 1-oxo-2-(methylamino)propyl | H | H | - | |
 | bk-6-MAPB | H | H | H | H | 1-oxo-2-(methylamino)propyl | H | - | |
 | 5-MBPB | H | H | H | 2-(methylamino)butyl | H | H | - | |
 | 6-MBPB | H | H | H | H | 2-(methylamino)butyl | H | - | |
 | 5-DBFPV | 2117405-32-8 | H | H | H | 1-oxo-2-(pyrrolidin-1-yl)pentyl | H | H | 2,3-dihydro |
 | 6-MeO-5-APDB | H | H | H | 2-aminopropyl | methoxy | H | 2,3-dihydro | |
 | F-2 | 99355-74-5 | methyl | H | H | methoxy | 2-aminopropyl | H | 2,3-dihydro |
 | F-22 | 952016-51-2 | dimethyl | H | H | methoxy | 2-aminopropyl | H | 2,3-dihydro |
 | 7-APB | 286834-86-4 | H | H | H | H | H | 2-aminopropyl | - |
 | DOI-2-HEMIFLY (4-I-5-MeO-7-APDB) | H | H | iodo | methoxy | H | 2-aminopropyl | 2,3-dihydro | |
 | Amiodarone | 1951-25-3 | propyl | 3,5-diiodo-4-(2-diethylamino-ethoxy)benzoyl | H | H | H | H | - |
 | 2C-B-FLY | 733720-95-1 | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | bromo | 2,3-dihydro |
 | 2C-B-DRAGONFLY | 260809-98-1 | H | H | 2-aminoethyl | furo[5,6-f] | - | bromo | - |
 | 2C-C-FLY | 1354633-83-2 | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | chloro | 2,3-dihydro |
 | 2C-I-FLY | 1354633-88-7 | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | iodo | 2,3-dihydro |
 | 2C-D-FLY | 1354634-07-3 | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | methyl | 2,3-dihydro |
 | 2C-E-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | ethyl | 2,3-dihydro | |
 | 2C-EF-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | 2-fluoroethyl | 2,3-dihydro | |
 | 2C-T-7-FLY | 1354633-05-8 | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | n-propylthio | 2,3-dihydro |
 | DOB-FLY | 219986-75-1 | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | bromo | 2,3-dihydro |
 | Bromo-DragonFLY | 502759-67-3 | H | H | 2-aminopropyl | furo[5,6-f] | - | bromo | - |
 | DOB-2-DRAGONFLY-5-BUTTERFLY | 1043541-82-7 | H | H | 2-aminopropyl | 5,6-dihydropyrano | - | bromo | - |
 | DOM-FLY | 748748-08-5 | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | methyl | 2,3-dihydro |
 | DOMOM-FLY [20] | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | methoxymethyl | 2,3-dihydro | |
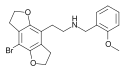 | 2C-B-FLY-NBOMe | 1335331-42-4 | H | H | 2-[(2-methoxybenzyl)-amino]ethyl | 5,6-dihydrofuro[5,6-f] | - | bromo | 2,3-dihydro |
 | 2C-B-DRAGONFLY-NBOH | 1335331-45-7 | H | H | 2-[(2-hydroxybenzyl)-amino]ethyl | furo[5,6-f] | - | bromo | - |
 | TFMFly | 780744-19-6 | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | trifluoromethyl | 2,3-dihydro |
 | Mescaline-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,4-b] | - | methoxy | 2,3-dihydro | |
 | YM-348 | 372163-84-3 | ethyl | H | 1-(2-aminopropyl)-pyrazol[4,5-f] | - | H | H | - |
 | 2-Desethyl-YM-348 | 748116-94-1 | H | H | 1-(2-aminopropyl)-pyrazol[4,5-f] | - | H | H | - |
Legislation
[edit]Substituted benzofurans saw widespread use as recreational drugs by being sold as research chemicals making them exempt from drug legislation. Many of the more common compounds were banned in the UK in June 2013 as temporary class drugs, while others have been made permanently illegal in various jurisdictions.[21][22][23]
See also
[edit]- 2-Aminoethoxydiphenyl borate (an unrelated compound also known as 2-APB)
- 2C-B-BUTTERFLY
- 5-IT
- AL-38022A
- IBF5MAP
- Jimscaline
- Substituted amphetamine
- Substituted benzothiophene
- Substituted cathinone
- Substituted methylenedioxyphenethylamine
- Substituted methoxyphenethylamine
- Substituted naphthylethylamine
- Substituted phenethylamine
- Substituted tryptamines
References
[edit]- ^ Dawood KM (September 2013). "Benzofuran derivatives: a patent review". Expert Opinion on Therapeutic Patents. 23 (9): 1133–56. doi:10.1517/13543776.2013.801455. PMID 23683135. S2CID 24425145.
- ^ Nevagi RJ, Dighe SN, Dighe SN (June 2015). "Biological and medicinal significance of benzofuran". European Journal of Medicinal Chemistry. 97: 561–81. doi:10.1016/j.ejmech.2014.10.085. PMID 26015069.
- ^ Khanam H (June 2015). "Bioactive Benzofuran derivatives: A review". European Journal of Medicinal Chemistry. 97: 483–504. doi:10.1016/j.ejmech.2014.11.039. PMID 25482554.
- ^ Nugteren-van Lonkhuyzen JJ, van Riel AJ, Brunt TM, Hondebrink L (December 2015). "Pharmacokinetics, pharmacodynamics and toxicology of new psychoactive substances (NPS): 2C-B, 4-fluoroamphetamine and benzofurans". Drug and Alcohol Dependence. 157: 18–27. doi:10.1016/j.drugalcdep.2015.10.011. PMID 26530501.
- ^ Liu C, Jia W, Qian Z, Li T, Hua Z (February 2017). "Identification of five substituted phenethylamine derivatives 5-MAPDB, 5-AEDB, MDMA methylene homolog, 6-Br-MDMA, and 5-APB-NBOMe". Drug Testing and Analysis. 9 (2): 199–207. doi:10.1002/dta.1955. PMID 26856255.
- ^ Barcelo B, Gomila I (2017). "Pharmacology and Literature Review Based on Related Death and Non-Fatal Case Reports of the Benzofurans and Benzodifurans Designer Drugs". Current Pharmaceutical Design. 23 (36): 5523–5529. doi:10.2174/1381612823666170714155140. PMID 28714411.
- ^ Halberstadt AL, Chatha M, Stratford A, Grill M, Brandt SD (January 2019). "Comparison of the behavioral responses induced by phenylalkylamine hallucinogens and their tetrahydrobenzodifuran ("FLY") and benzodifuran ("DragonFLY") analogs". Neuropharmacology. 144: 368–376. doi:10.1016/j.neuropharm.2018.10.037. PMC 6863604. PMID 30385253.
- ^ Roque Bravo R, Carmo H, Carvalho F, Bastos ML, Dias da Silva D (August 2019). "Benzo fury: A new trend in the drug misuse scene". Journal of Applied Toxicology. 39 (8): 1083–1095. doi:10.1002/jat.3774. PMID 30723925. S2CID 73433890.
- ^ Tomaszewski Z, Johnson MP, Huang X, Nichols DE (May 1992). "Benzofuran bioisosteres of hallucinogenic tryptamines". Journal of Medicinal Chemistry. 35 (11): 2061–4. doi:10.1021/jm00089a017. PMID 1534585.
- ^ Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE (November 1993). "Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine". Journal of Medicinal Chemistry. 36 (23): 3700–6. doi:10.1021/jm00075a027. PMID 8246240.
- ^ US patent 7045545, Karin Briner, Joseph Paul Burkhart, Timothy Paul Burkholder, Matthew Joseph Fisher, William Harlan Gritton, Daniel Timothy Kohlman, Sidney Xi Liang, Shawn Christopher Miller, Jeffrey Thomas Mullaney, Yao-Chang Xu, Yanping Xu, "Aminoalkylbenzofurans as serotonin (5-HT(2c)) agonists", published 19 January 2000, issued 16 May 2006
- ^ "Temporary class drug order report on 5-6APB and NBOMe compounds". UK Home Office. 4 June 2013. Retrieved 23 August 2016.
- ^ Stanczuk A, Morris N, Gardner EA, Kavanagh P (April 2013). "Identification of (2-aminopropyl)benzofuran (APB) phenyl ring positional isomers in internet purchased products". Drug Testing and Analysis. 5 (4): 270–6. doi:10.1002/dta.1451. PMID 23349125.
- ^ Nichols DE, Hoffman AJ, Oberlender RA, Riggs RM (February 1986). "Synthesis and evaluation of 2,3-dihydrobenzofuran analogues of the hallucinogen 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane: drug discrimination studies in rats". Journal of Medicinal Chemistry. 29 (2): 302–4. doi:10.1021/jm00152a022. PMID 3950910.
- ^ Nichols DE, Snyder SE, Oberlender R, Johnson MP, Huang XM (January 1991). "2,3-Dihydrobenzofuran analogues of hallucinogenic phenethylamines". Journal of Medicinal Chemistry. 34 (1): 276–81. doi:10.1021/jm00105a043. PMID 1992127.
- ^ Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE (July 1996). "Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups". Journal of Medicinal Chemistry. 39 (15): 2953–61. doi:10.1021/jm960199j. PMID 8709129.
- ^ Liu C, Jia W, Qian Z, Li T, Hua Z (February 2017). "Identification of five substituted phenethylamine derivatives 5-MAPDB, 5-AEDB, MDMA methylene homolog, 6-Br-MDMA, and 5-APB-NBOMe". Drug Testing and Analysis. 9 (2): 199–207. doi:10.1002/dta.1955. PMID 26856255.
- ^ Wagmann L, Brandt SD, Stratford A, Maurer HH, Meyer MR (February 2019). "Interactions of phenethylamine-derived psychoactive substances of the 2C-series with human monoamine oxidases" (PDF). Drug Testing and Analysis. 11 (2): 318–324. doi:10.1002/dta.2494. PMID 30188017. S2CID 52166076.
- ^ Wagmann L, Hempel N, Richter LH, Brandt SD, Stratford A, Meyer MR (October 2019). "Phenethylamine-derived new psychoactive substances 2C-E-FLY, 2C-EF-FLY, and 2C-T-7-FLY: Investigations on their metabolic fate including isoenzyme activities and their toxicological detectability in urine screenings". Drug Testing and Analysis. 11 (10): 1507–1521. doi:10.1002/dta.2675. PMID 31299701.
- ^ Feng Z, Mohapatra S, Klimko PG, Hellberg MR, May JA, Kelly C, Williams G, McLaughlin MA, Sharif NA. Novel benzodifuran analogs as potent 5-HT2A receptor agonists with ocular hypotensive activity. Bioorg Med Chem Lett. 2007 Jun 1;17(11):2998-3002. doi:10.1016/j.bmcl.2007.03.073 PMID 17419053
- ^ UK Home Office (5 March 2014). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government. Retrieved 23 August 2016.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 23 August 2016.
- ^ "指定薬物名称・構造式一覧(平成27年9月16日現在)" (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 23 August 2016.


 French
French Deutsch
Deutsch
