索米妥昔單抗 - 维基百科,自由的百科全书
 | |
| 单克隆抗体 | |
|---|---|
| 种类 | 完整抗体 |
| 目標 | 葉酸受體α |
| 臨床資料 | |
| 商品名 | Elahere |
| 其他名稱 | mirvetuximab soravtansine-gynx |
| 核准狀況 | |
| 给药途径 | 靜脈注射 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 识别信息 | |
| CAS号 | 1453084-37-1 |
| DrugBank | |
| UNII | |
| KEGG | |
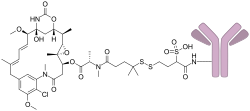
索米妥昔單抗(INN:Mirvetuximab soravtansine)用于治疗某些类型的卵巢癌、输卵管癌和原发性腹膜癌[4]。嚴格来说,它只适用于葉酸受體α(FRα)阳性且其他治疗方法無效的患者[4]。透過静脉注射給藥[4]。
常见的副作用包括视力问题、疲倦、肝脏问题、恶心、腹痛、白细胞减少症、周邊神經病變、腹泻、便秘、低血鎂症和贫血[4]。其他副作用包括可能非感染性肺炎[4]。孕期使用可能對婴儿有害[4]。它是针对 FRα 的单克隆抗体,藥物會與微管抑制剂結合[4]。
索米妥昔單抗于 2022 年在美国获取得医疗使用許可[4]。该药物在欧洲属于孤儿药[5]。
参考文獻
[编辑]- ^ Elahere- mirvetuximab soravtansine injection, solution. DailyMed. 18 November 2022 [4 December 2022].
- ^ Elahere EPAR. European Medicines Agency (EMA). 19 September 2024 [21 September 2024]. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Elahere PI. Union Register of medicinal products. 15 November 2024 [20 November 2024].
- ^ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Elahere- mirvetuximab soravtansine injection, solution. DailyMed. 18 November 2022 [4 December 2022]. (原始内容存档于4 December 2022). 互联网档案馆的存檔,存档日期4 December 2022.
- ^ Mirvetuximab soravtansine. SPS - Specialist Pharmacy Service. 27 March 2018 [16 December 2022]. (原始内容存档于25 June 2022). 互联网档案馆的存檔,存档日期25 June 2022.
延伸閱讀
[编辑]- Gonzalez-Ochoa E, Veneziani AC, Oza AM. Mirvetuximab Soravtansine in Platinum-Resistant Ovarian Cancer. Clinical Medicine Insights. Oncology. 2023, 17: 11795549231187264. PMC 10387675
 . PMID 37528890. doi:10.1177/11795549231187264.
. PMID 37528890. doi:10.1177/11795549231187264. - Porter RL, Matulonis UA. Mirvetuximab soravtansine for platinum-resistant epithelial ovarian cancer. Expert Review of Anticancer Therapy. 2023, 23 (8): 783–796. PMID 37458180. S2CID 259947077. doi:10.1080/14737140.2023.2236793.
外部連結
[编辑]- Clinical trial number NCT04296890 for "A Study of Mirvetuximab Soravtansine in Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression (SORAYA)" at ClinicalTrials.gov
- Clinical trial number NCT04209855 for "A Study of Mirvetuximab Soravtansine vs. Investigator's Choice of Chemotherapy in Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression (MIRASOL)" at ClinicalTrials.gov


 French
French Deutsch
Deutsch