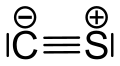Carbon monosulfide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name carbon monosulfide | |||
| Other names carbon(II) sulfide, thiocarbonyl, sulfidocarbon, methanidylidynesulfanium | |||
| Identifiers | |||
3D model (JSmol) | |||
| 1697516, 1918616 | |||
| ChEBI | |||
| ChemSpider | |||
| 648 | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| CS | |||
| Molar mass | 44.07 g·mol−1 | ||
| Appearance | reddish crystalline powder | ||
| insoluble | |||
| Related compounds | |||
Other anions | Carbon monoxide | ||
Other cations | Silicon monosulfide Germanium monosulfide Tin(II) sulfide Lead(II) sulfide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium.[1] The molecule resembles carbon monoxide with a triple bond between carbon and sulfur. The molecule is not intrinsically unstable, but it tends to polymerize in sunlight to a brown mass, as first discovered in 1868 and 1872.[2] The polymer is quite stable, decomposing a little at 360 °C to carbon disulfide. This tendency towards polymerization reflects the greater stability of C–S single bonds.
Polymers with the formula (CS)n have been reported,[3] and the formal dimer is ethenedithione. Also, CS has been observed as a ligand in some transition metal complexes.[citation needed]
The simplest carbon monosulfide synthesis decomposes carbon disulfide in a high-voltage AC arc.[4]
References
[edit]- ^ Wilson, R. W.; Penzias, A. A.; Wannier, P. G.; Linke, R. A. (1976). "Isotopic abundances in interstellar carbon monosulfide". Astrophysical Journal. 204 (pt 2): L135 – L137. Bibcode:1976ApJ...204L.135W. doi:10.1086/182072.
- ^
- Discovery in 1868: Loew, Oscar (1868). "Notiz über die Wirkung des Sonnenlichts auf Kohlenbisulfid", from Zeitschrift für Chemie, vol. 11 issue 4, p. 622 — via the Munich Digitization Center.
- History and subsequent elucidation of the polymer: Dewar, James; Owen Jones, Humphrey (1910). "Note on carbon monosulphide", from Proceedings of the Royal Society of London, Series A, volume 83 issue 564, pp. 408–413. doi:10.1098/rspa.1910.0029
- ^ Chou, J.-H.; Rauchfuss, T. B. (1997). "Solvatothermal Routes to Poly(Carbon Monosulfide)s Using Kinetically Stabilized Precursors" (PDF). Journal of the American Chemical Society. 119 (19): 4537–4538. doi:10.1021/ja970042w.
- ^ Moltzen, Ejner K.; Klabunde, Kenneth J.; and Senning, Alexander (1988). "Carbon monosulfide", from Chemical Reviews, vol. 88 issue 2, pp. 391-406. doi:10.1021/cr00084a003.


 French
French Deutsch
Deutsch