Cyclopropenone
 | |
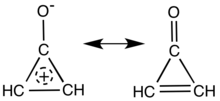 Main resonance structures of cyclopropenone. | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Cycloprop-2-en-1-one | |
| Other names Cyclopropenone, Cyclopropene-3-one | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C3H2O | |
| Molar mass | 54.048 g·mol−1 |
| Appearance | Colorless liquid |
| Melting point | −29 to −28 °C (−20 to −18 °F; 244 to 245 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Cyclopropenone is an organic compound with molecular formula C3H2O consisting of a cyclopropene carbon framework with a ketone functional group. It is a colorless, volatile liquid that boils near room temperature.[1] Neat cyclopropenone polymerizes upon standing at room temperature,[2] and chemical vendors typically supply it as an acetal.[3] The chemical properties of the compound are dominated by the strong polarization of the carbonyl group, which gives a partial positive charge with aromatic stabilization on the ring and a partial negative charge on oxygen. It is an aromatic compound.[4][5]
See also
[edit]References
[edit]- ^ R. Breslow, J. Pecoraro, T. Sugimoto "Cyclpropenone" Org. Synth. 1977, vol. 57, pp. 41. doi:10.15227/orgsyn.057.0041
- ^ Breslow, Ronald; Oda, Masaji (1972-06-01). "Isolation and characterization of pure cyclopropenone". Journal of the American Chemical Society. 94 (13): 4787–4788. doi:10.1021/ja00768a089. ISSN 0002-7863.
- ^ Elliott, Gregory I.; Boger, Dale L. "Cyclopropene, 3,3‑Dimethoxy". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00486.
Dimethyl cyclopropenone ketal is less stable than the corresponding cyclic ketal derivatives....A direct route to cyclopropenone is the hydrolysis of 3,3‑dimethoxycyclopropene.
- ^ "Experiments show cyclopropenone is aromatic". Chem. Eng. News. 61 (38): 33. 1983. doi:10.1021/cen-v061n038.p033.
- ^ Peart, Patricia A.; Tovar, John D. (2010). "Poly(cyclopropenone)s: Formal Inclusion of the Smallest Hückel Aromatic into π-Conjugated Polymers". J. Org. Chem. 76 (15): 5689–5696. doi:10.1021/jo101108f. PMID 20704438.


 French
French Deutsch
Deutsch