TIK-301
 | |
| Clinical data | |
|---|---|
| Other names | Beta-methyl-6-chloromelatonin; LY-156735; PD-6735 |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 1 hour |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
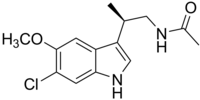
| Formula | C14H17ClN2O2 |
| Molar mass | 280.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
TIK-301 (LY-156735) is an agonist for the melatonin receptors MT1 and MT2 that is under development for the treatment of insomnia and other sleep disorders.[1] Its agonist action on MT1 and MT2 receptors in the suprachiasmatic nucleus in the brain enables its action as a chronobiotic. It is in the same class of melatonin receptor agonists as ramelteon and tasimelteon.
History and development
[edit]TIK-301 was first developed at Eli Lilly and Company in Indianapolis, Indiana, as LY-156735. In 2002, it was licensed by Phase 2 Discovery for further commercialization and worldwide development as PD-6735.[2][3] In July 2007, the open Investigational New Drug (IND) was transferred to Tikvah Therapeutics Inc. in Atlanta, GA by Phase II Discovery, where it was renamed to TIK-301. Currently, clinical trials are ongoing there.[4][5][6] Because it has been traded and sublicensed by multiple companies, it can referred to by all three names. Mostly recently and commonly, it is referred to as TIK-301.
TIK-301 was in phase II clinical trials in 2002.[2] In 2004, TIK-301 was designated an orphan drug by the FDA.[2][7]
Pharmacodynamics
[edit]TIK-301 is a high affinity nonselective MT1/MT2 agonist.[8] Studies show that it is more potent and more effective than melatonin. Its affinity for MT1 is similar to that of melatonin (pKi =10.38, Ki=81pM) and its affinity for MT2 is slightly higher (pKi=10.38, Ki= 42pM).[2][8][9][10][11] This enantiomer had higher affinity for the binding site compared to the racemic mixture.[2] The MT1/MT2 Ki ratio is 1.9.[12] This slight preference for MT2 receptor is common among melatonin derivatives with chlorine.[9][12] TIK-301's action on MT1 and MT2 receptors contributes to its sleep-promoting effects because melatonin's effects at these same receptors is linked with maintenance of normal-sleep wake cycle. TIK-301 was shown to be effective at promoting sleep at various doses; there is a positive dose response relationship between dose and reduction in sleep latency.[13] The EC50 of TIK-301 is 0.0479nM, compared to 0.063nM for melatonin.[8] It also acts as an antagonist at serotonin receptors 5-HT2B and 5-HT2C.[9]
Pharmacokinetics
[edit]TIK-301 is administered orally.[14] Compared to melatonin, it has nine times greater bioavailability and six times greater area under the curve (AUC), which means the body retains more of an administered dose.[13][14] TIK-301 was detected in blood plasma within 10 to 15 minutes of administration of a single oral dose and remains in a patient's system until 12 hours after the single dose.[13] Plasma concentrations increased rapidly and peaked at 1 hour after the dose, independent of dose size.[13] TIK-301's half-life is about 1 hour.[9][13] This extended half-life may be partially due to the chlorine in its structure.[11] Elimination constants depended on dose, 20 mg dose had a different elimination constant from all other doses above 35 mg.[13]
Treatment
[edit]TIK-301 is intended to be a take-as-need drug for primary insomnia, circadian rhythm disorders, depression, as well as sleep disorders in blind individuals and can be used to alleviate neuroleptic-induced tardive dyskinesia in schizophrenia patients.[15] In a phase I clinical trial, TIK-301 was shown to be effective as a chronobiotic at a dose of 5 mg/L, but not in lower doses.[14] In a phase II trial for primary insomnia, patients experienced objective and subjective improvements in sleep latency at 20 mg (31% improvement), 50 mg (32%) and 100 mg (41%) doses.[16] The sleep latency improvement at the 100 mg dose is comparable to FDA approved zolpidem's effects.[16] Surprisingly, it showed no such effects in healthy patients when taken before bed.[17] In a test of phase shifted circadian cycle, TIK-301 showed efficacy in readjusting phase shifts in all physiological systems.[14][18] While it has been shown to be effective in phase shifting circadian rhythm and reduced sleep latency, it has not been shown to help sleep maintenance, even at doses of 20 mg or 200 mg.[11]
In addition to a sleep aid, TIK-301 has been found useful in treating other disorders. Because of its affinity for serotonin receptors, it has potential to serve as a possible antidepressant drug, similar to agomelatine.[8][9][19] TIK-301 has also been considered for use in patients with mild cognitive impairment (MCI) because of sleep disorder prevalence.[15] TIK-301, as well as other melatonin agonists, has been reported to have potential in preventing or treating urinary incontinence, but have not been tested in humans for this purpose.[20][21] It is also seen as a potential therapeutic agent for spinal cord injury (SCI); in low doses (10 mg/kg) it was seen to be benefit in rats after SCI, but in higher doses (100 mg/kg), it proved toxic.[22]
Side effects
[edit]There were no major and serious side effects in phase I trials, and mild side effects such as diarrhea, conjunctivitis and laryngitis were observed in few cases.[14][16] Unlike benzodiazepines sleep medications, TIK-301's novel mode of action at melatonin receptors reduce many common side effects of sleep medications like dependency. In addition, TIK-301 had no latent, morning after psychomotor impairments.[16] A few patients reported cases of somnolence in clinical trials, which is consistent with the drug's soporific effects.[13]
Because of its receptor specific action, there are no associated changes in core body temperatures, heart rate or blood pressure as with other melatonin medications.[13][15][16][17]
References
[edit]- ^ "PD-6735, LY-156735,118702-11-7,C14-H17-Cl-N2-O2,N-[2(R)-(6-C--药物合成数据库". Archived from the original on 2009-09-27.
- ^ a b c d e Rivara S, Mor M, Bedini A, Spadoni G, Tarzia G (2008). "Melatonin receptor agonists: SAR and applications to the treatment of sleep-wake disorders". Current Topics in Medicinal Chemistry. 8 (11): 954–968. doi:10.2174/156802608784936719. PMID 18673165.
- ^ Mody S, Hu Y, Ho MK, Wong YH (2011-08-12). Frontiers in CNS Drug Discovery. Bentham eBooks. p. 606. ISBN 978-1-60805-159-5.
- ^ "Phase 2 Discovery, Inc. Acquires Drug in Clinical Development to Treat Sleep Disorders". Phase 2 Discovery, Inc. Acquires Drug in Clinical Development to Treat Sleep Disorders. Archived from the original on 2016-10-30. Retrieved 15 March 2015.
- ^ "Tikvah Therapeutics Signs Agreement to Develop and Commercialize LY156735, a Second Generation Melatonin Agonist, for Circadian Rhythm and Sleep Disorders". Nasdaq Global Newswire (Press release). 2007-08-29.
- ^ Kostiuk NV, Belyakova MB, Leshchenko DV, Zhigulina VV, Miniaev MV (2014). "Synthetic melatoninergic ligands: achievements and prospects". ISRN Biochemistry. 2014: 843478. doi:10.1155/2014/843478. PMC 4393004. PMID 25937968.
- ^ "Phase 2 Discovery, Inc. Receives Orphan Drug Designation From FDA For Synthetic Melatonin Analog PD-6735". BioSpace. 19 October 2005. Archived from the original on 4 March 2016.
- ^ a b c d Zlotos DP (2012). "Recent progress in the development of agonists and antagonists for melatonin receptors". Current Medicinal Chemistry. 19 (21): 3532–3549. doi:10.2174/092986712801323153. PMID 22680635.
- ^ a b c d e Carocci A, Catalano A, Sinicropi MS (2014). "Melatonergic drugs in development". Clinical Pharmacology. 6: 127–137. doi:10.2147/CPAA.S36600. PMC 4172069. PMID 25258560.
- ^ Zlotos DP, Jockers R, Cecon E, Rivara S, Witt-Enderby PA (April 2014). "MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential". Journal of Medicinal Chemistry. 57 (8): 3161–3185. doi:10.1021/jm401343c. PMID 24228714.
- ^ a b c Hardeland R, Poeggeler B (September 2012). "Melatonin and synthetic melatonergic agonists: actions and metabolism in the central nervous system". Central Nervous System Agents in Medicinal Chemistry. 12 (3): 189–216. doi:10.2174/187152412802430129. PMID 22640220.
- ^ a b Chan KH, Hu Y, Ho MK, Wong YH (2013). "Characterization of substituted phenylpropylamides as highly selective agonists at the melatonin MT2 receptor". Current Medicinal Chemistry. 20 (2): 289–300. doi:10.2174/0929867311320020009. PMID 23131177.
- ^ a b c d e f g h Mulchahey JJ, Goldwater DR, Zemlan FP (August 2004). "A single blind, placebo controlled, across groups dose escalation study of the safety, tolerability, pharmacokinetics and pharmacodynamics of the melatonin analog beta-methyl-6-chloromelatonin". Life Sciences. 75 (15): 1843–1856. doi:10.1016/j.lfs.2004.03.023. PMID 15302228.
- ^ a b c d e Nickelsen T, Samel A, Vejvoda M, Wenzel J, Smith B, Gerzer R (September 2002). "Chronobiotic effects of the melatonin agonist LY 156735 following a simulated 9h time shift: results of a placebo-controlled trial". Chronobiology International. 19 (5): 915–936. doi:10.1081/cbi-120014108. PMID 12405554. S2CID 10363563.
- ^ a b c Cardinali DP, Srinivasan V, Brzezinski A, Brown GM (May 2012). "Melatonin and its analogs in insomnia and depression" (PDF). Journal of Pineal Research. 52 (4): 365–375. doi:10.1111/j.1600-079x.2011.00962.x. PMID 21951153. S2CID 139480.
- ^ a b c d e Zemlan FP, Mulchahey JJ, Scharf MB, Mayleben DW, Rosenberg R, Lankford A (March 2005). "The efficacy and safety of the melatonin agonist beta-methyl-6-chloromelatonin in primary insomnia: a randomized, placebo-controlled, crossover clinical trial". The Journal of Clinical Psychiatry. 66 (3): 384–390. doi:10.4088/jcp.v66n0316. PMID 15766306.
- ^ a b Turek FW, Gillette MU (November 2004). "Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists". Sleep Medicine. 5 (6): 523–532. doi:10.1016/j.sleep.2004.07.009. PMID 15511698.
- ^ Doghramji K (August 2007). "Melatonin and its receptors: a new class of sleep-promoting agents". Journal of Clinical Sleep Medicine. 3 (5 Suppl): S17 – S23. doi:10.5664/jcsm.26932. PMC 1978320. PMID 17824497.
- ^ "PRESS RELEASE: Tikvah Therapeutics Signs Agreement with Phase 2 Discovery". fiercebiotech.com. 29 August 2007. Archived from the original on 2018-11-01. Retrieved 7 April 2015.
- ^ Rivara S, Pala D, Bedini A, Spadoni G (April 2015). "Therapeutic uses of melatonin and melatonin derivatives: a patent review (2012 - 2014)". Expert Opinion on Therapeutic Patents. 25 (4): 425–441. doi:10.1517/13543776.2014.1001739. hdl:11576/2623397. PMID 25579320. S2CID 19644131.
- ^ WO 2014010603, Watanabe K, Kawabata Y, Watanabe K, Yuyama N, Burgard S, Bruce E, "Pharmaceutical composition for treatment or prevention of stress urinary incontinence or mixed urinary incontinence, and screening method for compounds contained in the pharmaceutical composition", published 16 January 2014, assigned to Astellas Pharma Inc.
- ^ Fee DB, Swartz KR, Scheff N, Roberts K, Gabbita P, Scheff S (June 2010). "Melatonin-analog, beta-methyl-6-chloromelatonin, supplementation in spinal cord injury". Brain Research. 1340: 81–85. doi:10.1016/j.brainres.2010.04.047. PMID 20420812. S2CID 27784753.


 French
French Deutsch
Deutsch