Lysergamides
Amides of lysergic acid are collectively known as lysergamides or ergoamides,[1][2] and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors. Lysergamides contain an embedded tryptamine structure, and as a result can produce similar, often psychedelic, effects to those of the true tryptamines. [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17]
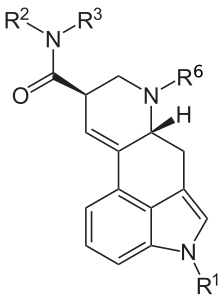
| Structure | Name | CAS number | R1 | R6 | R2 | R3 | Other |
|---|---|---|---|---|---|---|---|
 | LSA / LAA | 478-94-4 | H | CH3 | H | H | - |
 | DAM-57 | 4238-84-0 | H | CH3 | CH3 | CH3 | - |
 | Ergometrine (Ergonovine) | 60-79-7 | H | CH3 | CH(CH3)CH2OH | H | - |
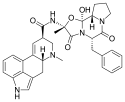 | Ergotamine | 113-15-5 | H | CH3 | -- | C17H18N2O4 | - |
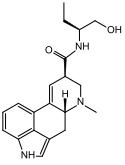 | Methergine | 113-42-8 | H | CH3 | CH(CH2CH3)CH2OH | H | - |
 | Methysergide | 361-37-5 | CH3 | CH3 | CH(CH2CH3)CH2OH | H | - |
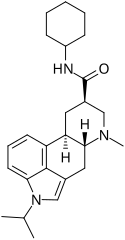 | Amesergide | 121588-75-8 | CH(CH3)2 | CH3 | C6H11 | H | - |
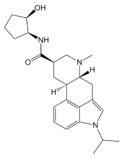 | LY-215840 | 137328-52-0 | CH(CH3)2 | CH3 | C5H8OH | H | - |
 | Cabergoline | 81409-90-7 | H | H2C=CH-CH2 | CONHCH2CH3 | CH2CH2CH2N(CH3)2 | - |
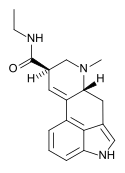 | LAE-32 | 478-99-9 | H | CH3 | CH2CH3 | H | - |
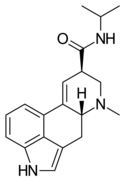 | LAiP | H | CH3 | CH(CH3)2 | H | - | |
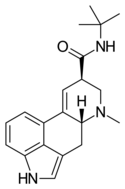 | LAtB | H | CH3 | C(CH3)3 | H | - | |
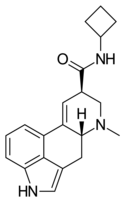 | LAcB | H | CH3 | (CH2)4 | H | - | |
 | Cepentil | H | CH3 | (CH2)5 | H | - | |
 | LSB | 137765-82-3 | H | CH3 | CH(CH3)CH2CH3 | H | - |
 | LSP | H | CH3 | CH(CH2CH3)CH2CH3 | H | - | |
 | DAL | H | CH3 | H2C=CH-CH2 | H2C=CH-CH2 | - | |
 | MIPLA | 100768-08-9 | H | CH3 | CH(CH3)2 | CH3 | - |
 | EIPLA | H | CH3 | CH(CH3)2 | CH2CH3 | - | |
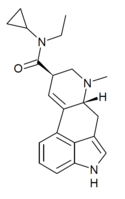 | ECPLA | H | CH3 | C3H5 | CH2CH3 | - | |
 | ETFELA | H | CH3 | CH2CF3 | CH2CH3 | - | |
 | LAMPA | 40158-98-3 | H | CH3 | CH2CH2CH3 | CH3 | - |
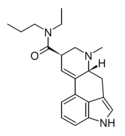 | EPLA | H | CH2CH3 | CH2CH2CH3 | CH3 | - | |
 | LSD / LAD | 50-37-3 | H | CH3 | CH2CH3 | CH2CH3 | - |
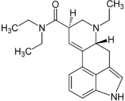 | ETH-LAD | 65527-62-0 | H | CH2CH3 | CH2CH3 | CH2CH3 | - |
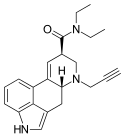 | PARGY-LAD | H | HC≡C−CH2 | CH2CH3 | CH2CH3 | - | |
 | AL-LAD | 65527-61-9 | H | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - |
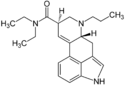 | PRO-LAD | 65527-63-1 | H | CH2CH2CH3 | CH2CH3 | CH2CH3 | - |
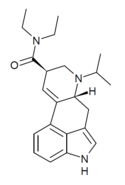 | IP-LAD | H | CH(CH3)2 | CH2CH3 | CH2CH3 | - | |
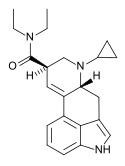 | CYP-LAD[18] | H | C3H5 | CH2CH3 | CH2CH3 | - | |
 | BU-LAD | 96930-87-9 | H | CH2CH2CH2CH3 | CH2CH3 | CH2CH3 | - |
 | FLUORETH-LAD[19] | H | CH2CH2F | CH2CH3 | CH2CH3 | - | |
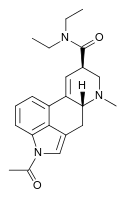 | ALD-52 | 3270-02-8 | COCH3 | CH3 | CH2CH3 | CH2CH3 | - |
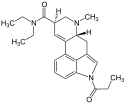 | 1P-LSD | 2349358-81-0 | COCH2CH3 | CH3 | CH2CH3 | CH2CH3 | - |
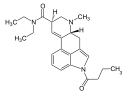 | 1B-LSD | 2349376-12-9 | COCH2CH2CH3 | CH3 | CH2CH3 | CH2CH3 | - |
 | 1V-LSD | CO(CH2)3CH3 | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1H-LSD[20] | CO(CH2)4CH3 | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1DD-LSD | CO(CH2)10CH3 | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1cP-LSD[21] | COC3H5 | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1D-LSD | COC4H5(CH3)2 | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1T-LSD | COC4H3S | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1S-LSD | CO(CH2)2Si(CH3)3 | CH3 | CH2CH3 | CH2CH3 | - | |
 | 1P-AL-LAD | COCH2CH3 | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - | |
 | 1cP-AL-LAD | COC3H5 | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - | |
 | 1T-AL-LAD[22] | COC4H3S | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - | |
 | 1P-ETH-LAD | COCH2CH3 | CH2CH3 | CH2CH3 | CH2CH3 | - | |
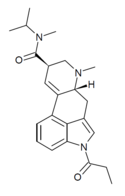 | 1P-MIPLA | COCH2CH3 | CH3 | CH(CH3)2 | CH3 | - | |
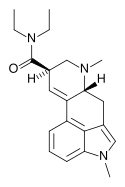 | MLD-41 | 4238-85-1 | CH3 | CH3 | CH2CH3 | CH2CH3 | - |
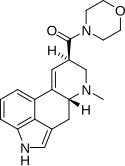 | LSM-775 | 4314-63-0 | H | CH3 | CH2CH2-O-CH2CH2 | - | |
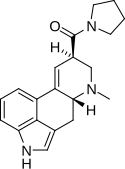 | LPD-824 | 2385-87-7 | H | CH3 | (CH2)4 | - | |
 | LSD-Pip | 50485-23-9 | H | CH3 | (CH2)5 | - | |
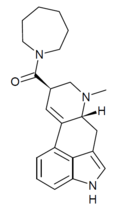 | LSD-Azapane | H | CH3 | (CH2)6 | - | ||
 | LA-SS-Az | 470666-31-0 | H | CH3 | CH2(CHCH3)2CH2 | - | |
 | 2-Bromo-LSD | 478-84-2 | H | CH3 | CH2CH3 | CH2CH3 | 2-Br |
 | 12-Methoxy-LSD[23] | 50484-99-6 | H | CH3 | CH2CH3 | CH2CH3 | 12-OMe |
 | 13-Fluoro-LSD[24] | H | CH3 | CH2CH3 | CH2CH3 | 13-F | |
 | 14-Hydroxy-LSD[25] | H | CH3 | CH2CH3 | CH2CH3 | 14-OH | |
See also
[edit]References
[edit]- ^ Jamieson CS, Misa J, Tang Y, Billingsley JM (2021-04-29). "Biosynthesis and synthetic biology of psychoactive natural products". Chemical Society Reviews. 50 (12): 6950–7008. doi:10.1039/D1CS00065A. ISSN 0306-0012. PMC 8217322. PMID 33908526.
“There are three main ergot alkaloid classes, clavines, ergoamides (lysergamides), and ergopeptides, with 3 belonging to the ergoamide class.” 2.5 Lysergic acid and LSD, p. 6970 - ^ Wong G, Lim LR, Tan YQ, Go MK, Bell DJ, Freemont PS, et al. (2022-02-07). "Reconstituting the complete biosynthesis of D-lysergic acid in yeast". Nature Communications. 13 (1): 712. Bibcode:2022NatCo..13..712W. doi:10.1038/s41467-022-28386-6. ISSN 2041-1723. PMC 8821704. PMID 35132076
“The ergot alkaloids are broadly classified into three groups—the clavines, ergoamides, and the ergopeptines, all of which are distinguished by the different modifications appended to the core ergoline structure.” Results and discussion / Biosynthetic resolution of the ergot alkaloid pathway{{cite journal}}: CS1 maint: postscript (link) - ^ Hofmann A (June 1959). "Psychotomimetic drugs; chemical and pharmacological aspects". Acta Physiologica et Pharmacologica Neerlandica. 8: 240–58. PMID 13852489.
- ^ US patent 2997470, Pioch RP, "LYSERGIC ACID AMIDES", published 1956-03-05, issued 1961-08-22
- ^ Hoffman AJ, Nichols DE (September 1985). "Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives". Journal of Medicinal Chemistry. 28 (9): 1252–1255. doi:10.1021/jm00147a022. PMID 4032428.
- ^ Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE (March 1994). "Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives". Pharmacology, Biochemistry, and Behavior. 47 (3): 667–673. doi:10.1016/0091-3057(94)90172-4. PMID 8208787. S2CID 16490010.
- ^ Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB (April 1995). "LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors". Psychopharmacology. 118 (4): 401–409. doi:10.1007/BF02245940. PMID 7568626. S2CID 21484356.
- ^ Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (September 2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". Journal of Medicinal Chemistry. 45 (19): 4344–4349. doi:10.1021/jm020153s. PMID 12213075.
- ^ Schiff PL (October 2006). "Ergot and its alkaloids". American Journal of Pharmaceutical Education. 70 (5): 98. doi:10.5688/aj700598 (inactive 2024-11-22). PMC 1637017. PMID 17149427.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
- ^ Nichols DE (2017). "Chemistry and Structure-Activity Relationships of Psychedelics". Current Topics in Behavioral Neurosciences. 36: 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. PMID 28401524.
- ^ Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, et al. (September 2016). "Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD)". Drug Testing and Analysis. 8 (9): 891–902. doi:10.1002/dta.1884. PMC 4829483. PMID 26456305.
- ^ Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, et al. (January 2017). "Return of the lysergamides. Part II: Analytical and behavioural characterization of N6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2'S,4'S)-lysergic acid 2,4-dimethylazetidide (LSZ)". Drug Testing and Analysis. 9 (1): 38–50. doi:10.1002/dta.1985. PMC 5411264. PMID 27265891.
- ^ Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Stratford A, et al. (October 2017). "Return of the lysergamides. Part III: Analytical characterization of N6 -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD)". Drug Testing and Analysis. 9 (10): 1641–1649. doi:10.1002/dta.2196. PMC 6230477. PMID 28342178.
- ^ Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, et al. (February 2018). "Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775)". Drug Testing and Analysis. 10 (2): 310–322. doi:10.1002/dta.2222. PMC 6230476. PMID 28585392.
- ^ Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Dowling G, et al. (August 2019). "Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B-LSD)". Drug Testing and Analysis. 11 (8): 1122–1133. doi:10.1002/dta.2613. PMC 6899222. PMID 31083768.
- ^ Halberstadt AL, Klein LM, Chatha M, Valenzuela LB, Stratford A, Wallach J, et al. (February 2019). "Pharmacological characterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide (ECPLA)". Psychopharmacology. 236 (2): 799–808. doi:10.1007/s00213-018-5055-9. PMC 6848745. PMID 30298278.
- ^ Kruegel AC. Novel Ergolines and Methods of Treating Mood Disorders. Patent WO 2022/226408
- ^ WO 2022/008627, Grill M, "Improved Method for the Production of Lysergic Acid Diethylamide (LSD) and Novel Derivatives thereof."
- ^ Brandt SD, Kavanagh PV, Gare S, Stratford A, Halberstadt AL. Analytical and behavioral characterization of 1-hexanoyl-LSD (1H-LSD). Drug Test Anal. 2024 Jul 4. doi:10.1002/dta.3767 PMID 38965834
- ^ Brandt SD, Kavanagh PV, Westphal F, Stratford A, Odland AU, Klein AK, et al. (June 2020). "Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1-cyclopropanoyl-d-lysergic acid diethylamide (1CP-LSD)". Drug Testing and Analysis. 12 (6): 812–826. doi:10.1002/dta.2789. PMC 9191646. PMID 32180350.
- ^ Okada Y, Segawa H, Yamamuro T, Kuwayama K, Tsujikawa K, Kanamori T, et al. (June 2024). "Synthesis and analytical characterization of 1-(2-thienoyl)-6-allyl-nor-d-lysergic acid diethylamide (1T-AL-LAD)". Drug Testing and Analysis. doi:10.1002/dta.3747. PMID 38922764.
- ^ Usdin E, Efron DH. Psychotropic Drugs and Related Compounds. (1972) ASIN B002X3CDIY
- ^ WO 2021/076572, Olson DE, et al., "Ergoline-like compounds for promoting neural plasticity"
- ^ Libânio Osório Marta RF (August 2019). "Metabolism of lysergic acid diethylamide (LSD): an update". Drug Metabolism Reviews. 51 (3): 378–387. doi:10.1080/03602532.2019.1638931. PMID 31266388.


 French
French Deutsch
Deutsch